INTRODUCTION
METHODS
Materials
Animal care and isolation of VSMCs
Cell viability assay
Scratch wound healing assay
Boyden chamber assay
Immunoblotting assay
Statistical analysis
RESULTS
Inhibitory effects of AUY922 on PDGF-BB-induced proliferation of VSMCs
 | Fig. 1Effect of AUY922 on platelet-derived growth factor (PDGF)-BB-induced vascular smooth muscle cells (VSMCs) proliferation.(A) Structure of AUY922. Molecular weight: 465.5 g/ml. (B) VSMCs were treated with 0.005% sodium dodecyl sulfate (vehicle, veh), 1% Triton X-100, and 5 nM AUY922 for 24 h. The cell morphologies were captured using the microscopy. Magnification, ×200. (C) VSMCs (2 × 104 cells/ml) were seeded into a 96-well plate for 24 h. Cells were exposed to difference concentrations of AUY922 (1, 2, 3, 5, 10, 20, 30, and 50 nM) for 24 h. Cell viability was determined using 2,3-Bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide. (D) VSMCs were incubated with serum-free media for 24 h and treated with or without PDGF-BB (10 ng/ml) and AUY922 (1, 2, 3, and 5 nM) for 24 h. The viability at the quiescent state is expressed as 100%. Data are expressed as means ± standard deviation. Different superscript letters indicate significant differences based on Tukey's multiple range test (p < 0.05).
|
AUY922 attenuates the migration of PDGF-BB-induced VSMCs
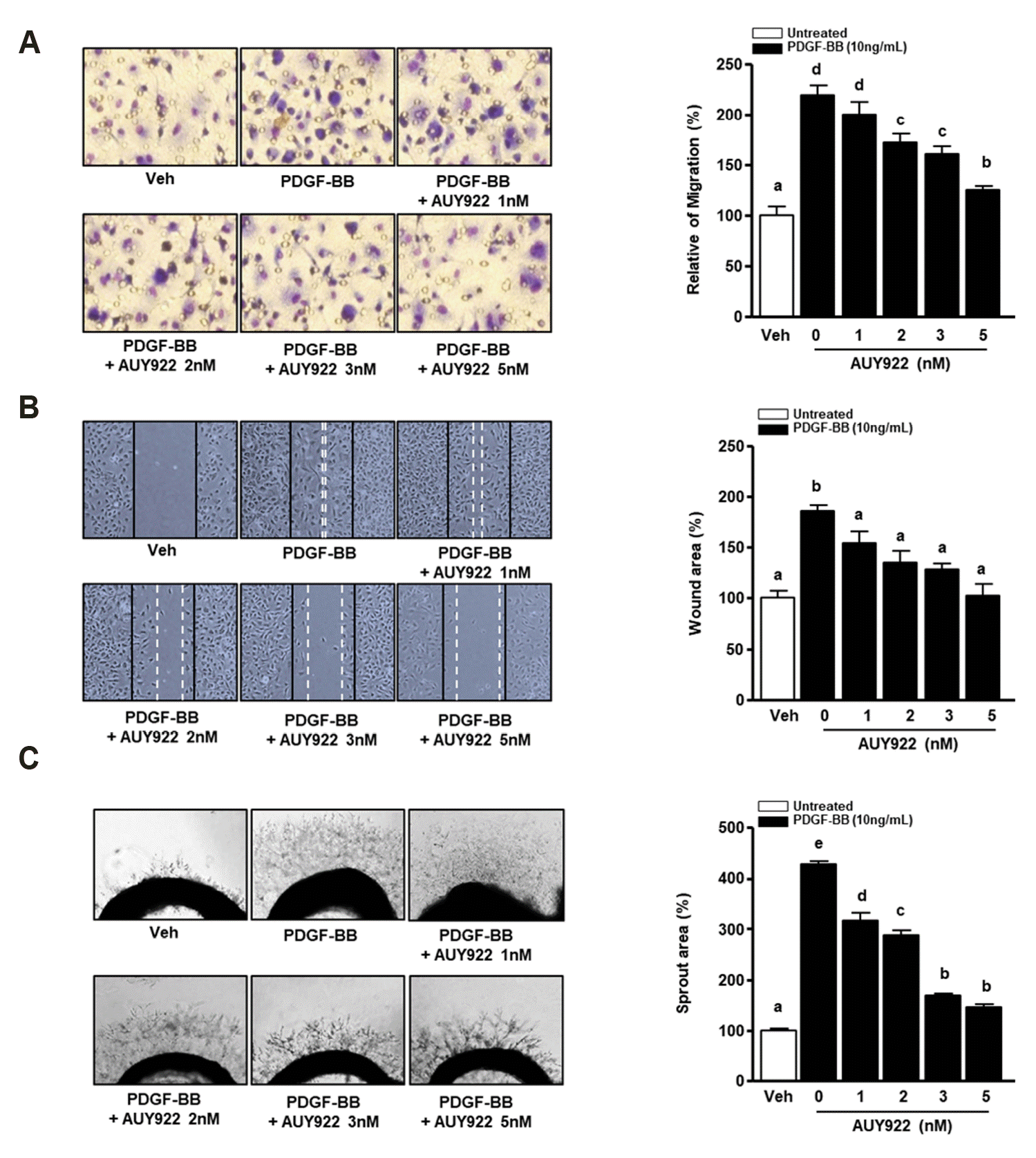 | Fig. 2Effect of AUY922 on platelet-derived growth factor (PDGF)-BB-induced in vitro and ex vivo models.(A) Vascular smooth muscle cells (VSMCs) treated with AUY922 (1, 2, 3, and 5 nM) for 90 min at 37°C. Cells were stained using a Diff-Quik Kit. (B) Cells were scratched with 200-µl tips and co-treated with PDGF-BB (10 ng/ml) and AUY922 (1, 2, 3, and 5 nM) for 24 h. The black solid line shows the scratch at 0 h and the white dotted line shows the scratch at 24 h. The wound healing area is expressed as 100%. Data are expressed as means ± standard deviation (SD). *Significant difference compared to the PDGF-BB group (p < 0.05). (C) Rat aortas were sliced to obtain 1-mm sections and embedded in Matrigel. The aortic rings were treated with or without PDGF-BB (10 ng/ml) and AUY922 (1, 2, 3, and 5 nM) for 4 days. The sprout area in the vehicle (veh) group is expressed as 100%. The photographs were captured using the microscopy. Magnification, ×200. Data are expressed as means ± SD. Different superscript letters indicate significant differences based on Tukey's multiple range test (p < 0.05).
|
AUY922 reduces AKT and ERK1/2 phosphorylation in PDGF-BB-stimulated VSMCs
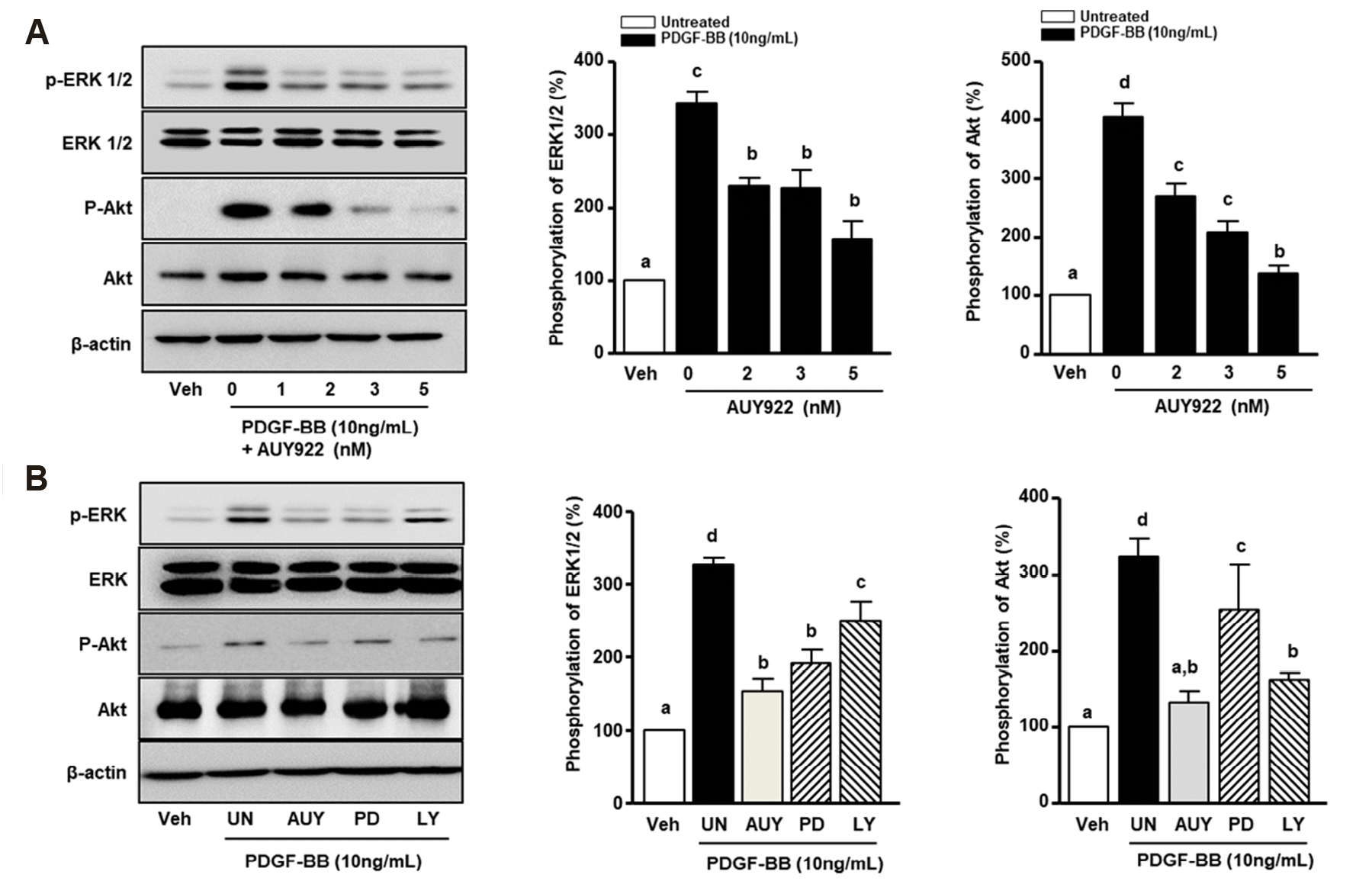 | Fig. 3Western blot analysis of extracellular signal-regulated kinase (ERK) 1/2 and Akt phosphorylation.(A) Vascular smooth muscle cells (VSMCs) were incubated in serum-free media and stimulated with platelet-derived growth factor (PDGF)-BB (10 ng/ml) and AUY922 (2, 3, and 5 nM) for 15 min. The cell lysates were separated by SDS-PAGE. Protein expression was detected using specific antibodies, such as anti-phosphorylated (p) ERK1/2, p-ERK1/2, Akt, and p-Akt. Graphs show the intensity of p-ERK1/2 and p-Akt, respectively. (B) VSMCs were stimulated in the presence or absence of PDGF-BB (10 ng/ml), PD98059 (30 μM), LY294002 (20 μM), and AUY922 (5 nM) for 30 min. Cells were lysed and protein expression was analyzed by western blotting. Data in graphs correspond to the images on the left. The viability at the quiescent state is expressed as 100%. Data are expressed as means ± standard deviation. Different superscript letters indicate significant differences based on Tukey's multiple range test (p < 0.05). Veh, vehicle.
|
Role of AUY922 in PDGF-BB-stimulated VSMCs
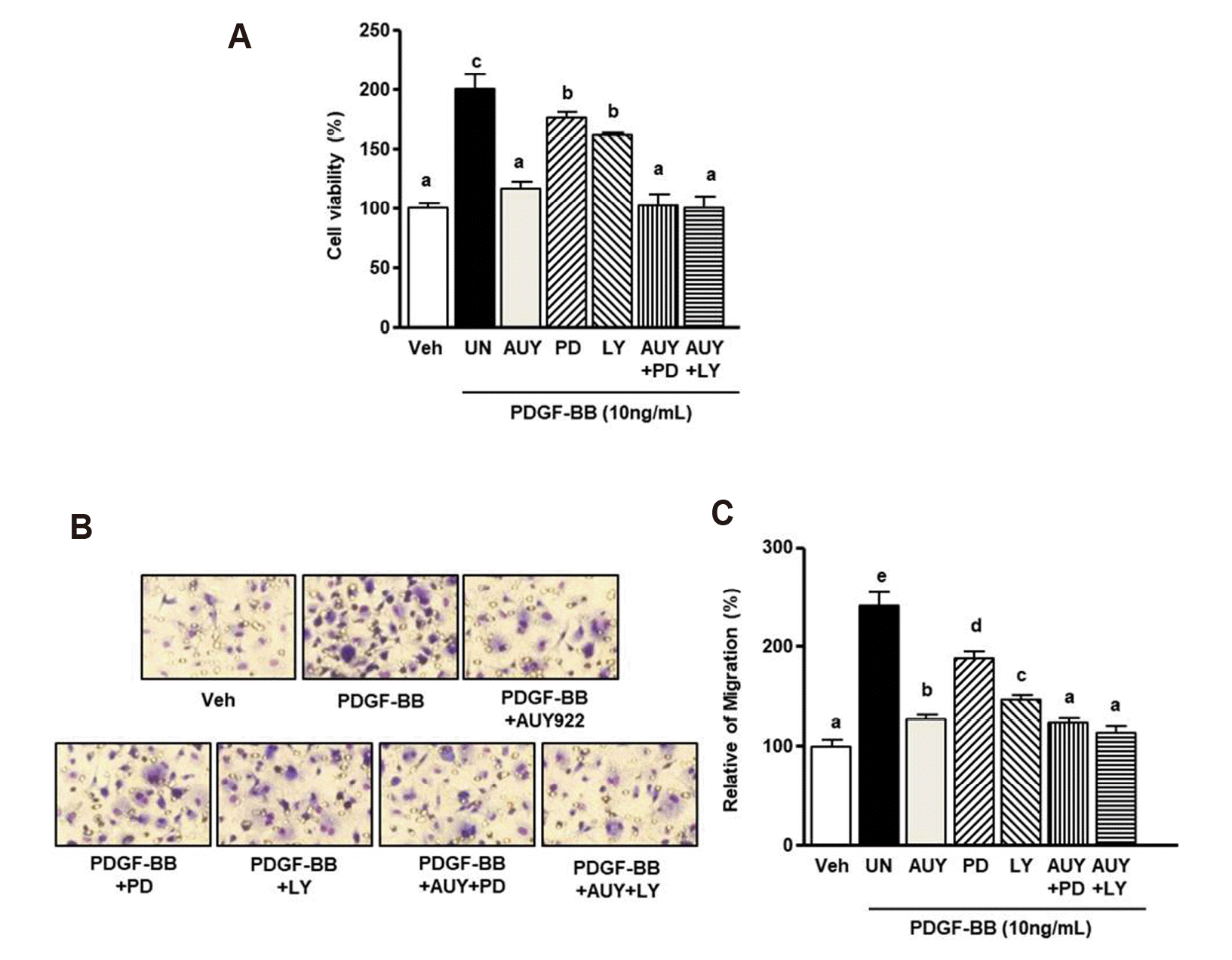 | Fig. 4Extracellular signal-regulated kinase (ERK) 1/2 and Akt inhibitors decreased platelet-derived growth factor (PDGF)-BB-induced increases in vascular smooth muscle cells (VSMC) proliferation and migration.(A) VSMCs were treated with PDGF-BB, PD98059 (30 μM), LY294002 (20 μM), and AUY922 (5 nM) for 24 h. The proliferation of VSMCs was determined by the XTT reagent. The viability at the quiescent state is expressed as 100%. (C) Graphs were obtained by Panel B. Data are expressed as means ± standard deviation (SD). *Significant difference compared to the PDGF-BB group (p < 0.05). (B) VSMCs (1 × 106 cells/ml) incubated with PD98059 (30 μM), LY294002 (20 μM), and AUY922 (5 nM) in the upper chamber and co-stimulated with PDGF-BB (10 ng/ml) in the lower chamber for 90 min. Magnification, ×200. Data are expressed as means ± SD. Different superscript letters indicate significant differences based on Tukey's multiple range test (p < 0.05). Veh, vehicle.
|




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download