INTRODUCTION
Anti-neutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) is a rare autoimmune disease (AID) involving the small vessels and mainly affecting older patients [
1]. Three distinct groups of diseases―microscopic polyangiitis (MPA), granulomatosis with polyangiitis (GPA), and eosinophilic polyangiitis (EGPA)―comprise AAV, which is typically associated with necrotising intravascular inflammation [
2,
3]. In AAV, multiple aetiologies, including environmental factors, genetics, and infection, are proposed to contribute to the pathogenesis; however, growing evidence indicates that immune dysregulation is crucial in provoking and perpetuating the inflammatory response [
4]. Interestingly, it has been identified that the trigger of innate and adaptive immunity could cause a shift in metabolic activity [
5]. Meanwhile, even though remarkable advances have recently been made in the understanding of AAV, the clinical and laboratory aspects associated with inflammation remain to be better investigated.
Compelling evidence now indicates that patients with AIDs show altered lipid metabolism, which could differ based on the underlying disease [
6]. In rheumatoid arthritis (RA), it is described that patients with active disease exhibit a “lipid paradox”, in which reduced total, high-density lipoprotein (HDL), and low-density lipoprotein (LDL) cholesterol are present in proportion to the degree of inflammation [
7]. Similarly, it has been shown that patients with systemic lupus erythematosus (SLE) present with decreased total and HDL cholesterol, whereas triglyceride, total, and LDL cholesterol levels are elevated at the time of diagnosis [
8]. Of note, it was demonstrated that low HDL cholesterol was associated with higher disease activity in SLE, whereas alterations of triglyceride levels indicated changes in disease activity, emphasising that lipid profiles could be affected by inflammation, and could be markers of disease severity in AIDs. However, owing to the rarity of the disease, the lipid profiles in patients with AAV and their relationship with disease activity has not yet been well determined. Therefore, the objective of this study was to investigate whether changes of lipid profiles correlate with disease severity in AAV.
DISCUSSION
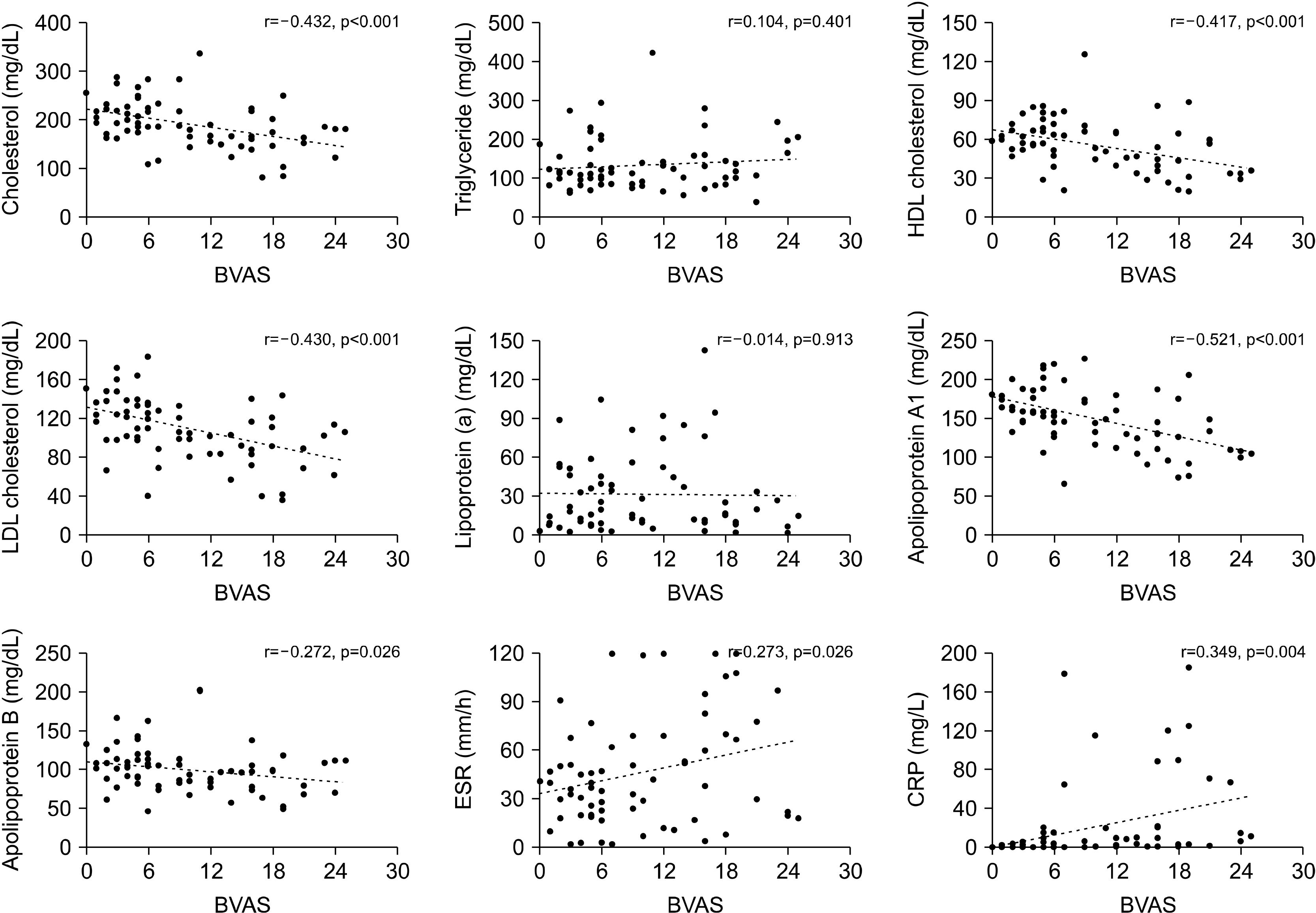
Although research concerning lipid profiles and its clinical significance in patients with AAV is scarce, a recent publication by Wallace and colleagues [
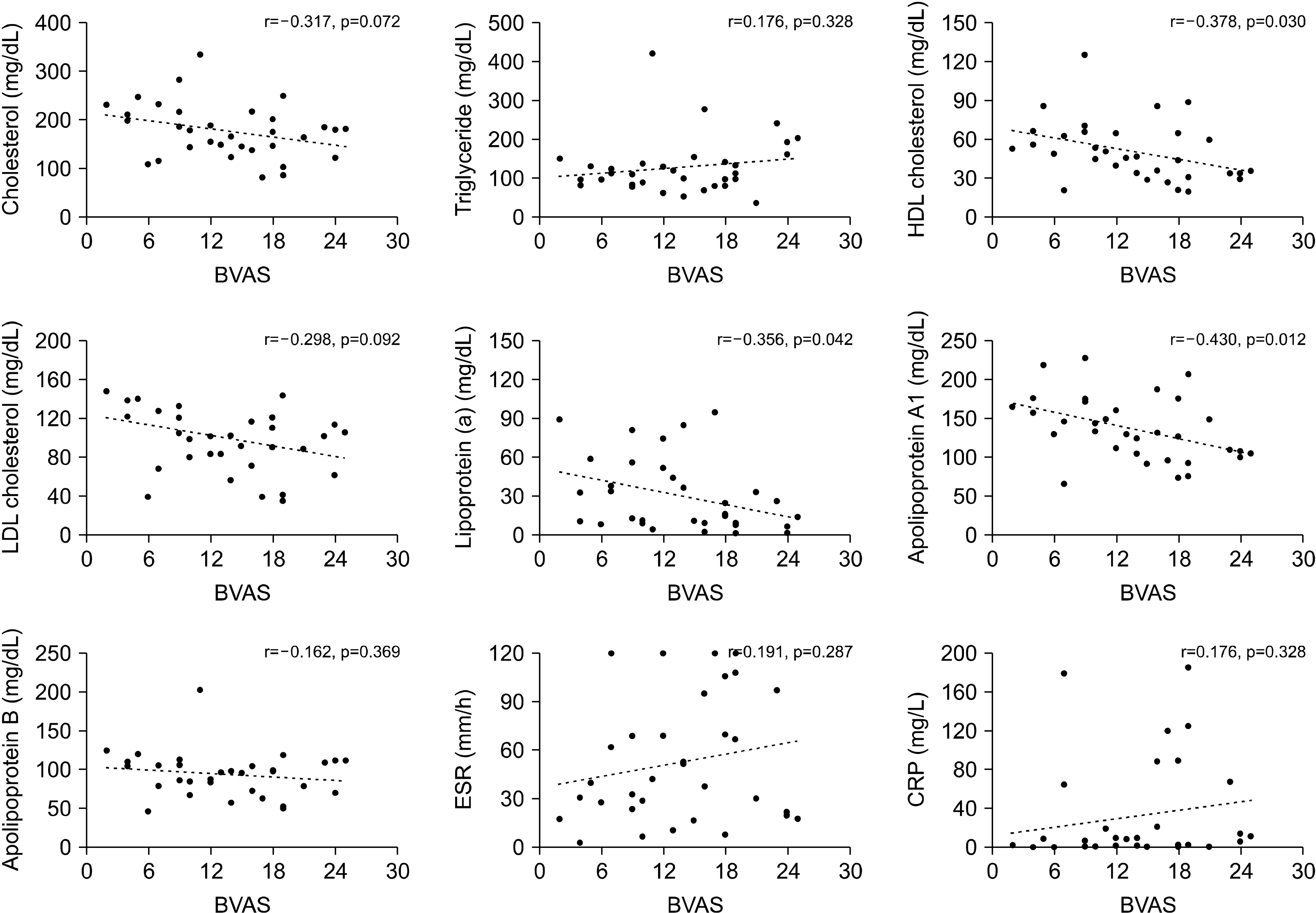
14] analysed lipid levels in patients’ sera from the Rituximab for ANCA-Associated Vasculitis (RAVE) trial. This study revealed that lipid levels of total and LDL cholesterol and of apolipoproteins A1 and B increase after treatment in patients with newly diagnosed AAV, implying that the decrease of lipid profiles is relevant to a higher inflammatory burden in AAV. These findings were reproduced in our study, as BVAS, which estimates patients’ assessment of global disease activity in AAV, was inversely correlated with total and LDL cholesterol and apolipoprotein A1 and B levels. This confirmed that escalated inflammation directly contributes to the decrease of lipids. However, in our study, the HDL cholesterol level was also associated with disease activity in AAV. Of special interest, we demonstrated that only HDL cholesterol and apolipoprotein A1 were significantly associated with BVAS, even in patients with new-onset disease. Moreover, the absolute value of the correlation coefficient between BVAS and apolipoprotein A1 was the highest among the lipid profiles and acute phase reactants, indicating that apolipoprotein A1 could be a surrogate marker of disease activity in AAV.
It is unclear why apolipoprotein A1 is most relevant to disease severity in AAV within the lipid profiles investigated. However, this could be partly attributed to the anti-inflammatory properties of HDL cholesterol, and due to apolipoprotein A1’s critical role in modulating the function of HDL cholesterol. HDL cholesterol is traditionally considered “good” cholesterol because it removes cholesterol from local tissues; it also transports excess cholesterol to the liver, in a process known as reverse cholesterol transport, thereby exerting protective effects in the development of atherosclerosis and cardiovascular diseases [
15,
16]. In contrast, HDL cholesterol has also been acknowledged to have anti-inflammatory characteristics, as it suppresses antigen presenting cells and T-cell activation [
17,
18]. In line with these findings, a previous study indicated that infusion of HDL cholesterol could alleviate arthritis in an experimental model of RA [
19]. Meanwhile, apolipoprotein A1 is a major protein that constitutes nearly 70% of proteins in HDL, and facilitates the transfer of cholesterol to HDL from tissues, and the transport of HDL cholesterol within the circulation to the liver [
20]. Given that apolipoprotein A1 is essential to the function of HDL cholesterol, the decrease of apolipoprotein A1 may be most significantly related to higher disease activity in AIDs, particularly AAV. Interestingly, a previous study has also shown that apolipoprotein A1 and HDL cholesterol levels decrease in the sera of patients with inflammatory arthritis compared to controls, which supports our hypothesis [
21]. Moreover, it was demonstrated in the same study that apolipoprotein A1 is decreased in the blood and increased in the inflamed joints in RA, indicating that the decrease of apolipoprotein A1 in the circulation could be closely linked to the inflammatory process.
Similarly, when we analysed the association between lipid profiles, acute phase reactants, and the clinical scores comprising BVAS, it was demonstrated that apolipoprotein A1 was significantly correlated with general, pulmonary, and renal manifestation scores. Notably, apolipoprotein A1 had the highest correlation with the renal manifestation score among the laboratory variables included. In addition, even though CRP, a representative acute phase reactant used to assess the degree of inflammation, had a higher correlation with general manifestation scores than did apolipoprotein A1, apolipoprotein A1 better correlated with the renal manifestation scores than did CRP. Taken together, it is suggested that the differential expression of lipid profiles could be helpful in predicting organ involvement patterns in AAV.
While the three representative diseases of MPA, GPA, and EGPA are traditionally known to comprise a group of AAV, it is increasingly understood that a distinct feature could be present according to the ANCA serostatus and the diagnosis [
22,
23]. In addition, it was described that total and LDL cholesterol and apolipoprotein B levels are significantly elevated following treatment in PR3-ANCA-positive patients, but not in MPO-ANCA positive patients [
14]. However, our observations revealed no differences in lipid profiles regarding diagnosis or ANCA serostatus. Although the difference in study design could have affected the discordant result, this discrepancy could be also accounted for by the fact that PR3-ANCA is detected more frequently than is MPO-ANCA in AAV patients in the Western world, and is associated with higher disease relapse [
24-
26]. In contrast, MPO-ANCA-positive AAV is more common than is PR3-ANCA positive AAV in Asia, and its presence is associated with higher disease activity in Korean patients [
24,
27]. Therefore, increased inflammatory burdens, rather than disease subtypes or ANCA types, may be an important factor influencing lipid profiles in patients with AAV.
Besides disease activity, medications treating dyslipidaemia, as well as glucocorticoids and immunosuppressive agents, could also affect patients’ lipid profiles [
28,
29]. Accordingly, in the study design, we only included patients with AAV not taking lipid-lowering agents, and the immunosuppressants that were being administered were also compared between patients with high and low disease activity. However, our findings indicate that the use of medications was comparable between the two groups, implying that immunosuppressant use did not significantly impact the lipid profiles of our study population.
Several inherent limitations are present in this study. First, the number of patients enrolled in this study was relatively small, which could have affected data inter-pretation. Furthermore, as we defined the patients into high disease activity and low disease activity group according to the BVAS cut-off of 12, this could have influenced in the study results. Second, as this study was a cross-sectional study, we were not able to evaluate the changes of lipid profiles according to the alterations of disease activity. In addition, due to the lack of a control group, a direct comparison of lipid profiles could not be made with that of the normal population. Third, data of lifestyle habits such as alcohol ingestion and smoking, which could impact in the lipid profiles, was not collected in this study. Fourth, the present study did not provide the mechanism leading to dysregulated lipid profiles. Hence, further exploration is necessary to understand the pathways that lead to aberrant lipid levels in AAV. Fifth, it is unclear whether the decrease in lipid profiles that were observed in this study is a general phenomenon in chronic inflammatory diseases or is a specific finding for AAV, and should be addressed through future studies.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download