Abstract
The World Health Organization classified rectal neuroendocrine tumors (NETs) as malignant in 2010 owing to their distant metastasis potential. On the other hand, in cases of small rectal NETs (<10 mm), which have a low risk of metastasis, endoscopic removal is the first-line therapeutic option, and regular surveillance is not recommended. The authors report a case of a small, well-differentiated rectal NET, which recurred as multiple hepatic metastases 5 years after apparent complete removal using endoscopic methods.
Go to : 
Generally, neuroendocrine tumors (NETs) occurring in the rectum are less than 10 mm in size and exhibit well-differentiated microscopic features. Most “small” lesions grow slowly compared to adenocarcinoma and can be removed easily using endoscopic methods, which has yielded favorable prognoses.1,2 On the other hand, there is a risk of metastasis to other organs because of the potential for invasive progression. Therefore, the World Health Organization and the American Joint Committee on Cancer classified rectal NETs as malignant diseases.3,4 Many international clinical guidelines have suggested algorithms for treatment and post-treatment surveillance tailored to each disease status, but there are always unexpected exceptions in real-world settings. This paper reports a case of small rectal NET that was treated successfully using an endoscopic en-bloc resection. In follow-up studies, however, multiple hepatic metastases were found without a local recurrence. This study was approved by the Institutional Research Ethics Board of the Catholic University of Korea (VC19ZISE0304).
Go to : 
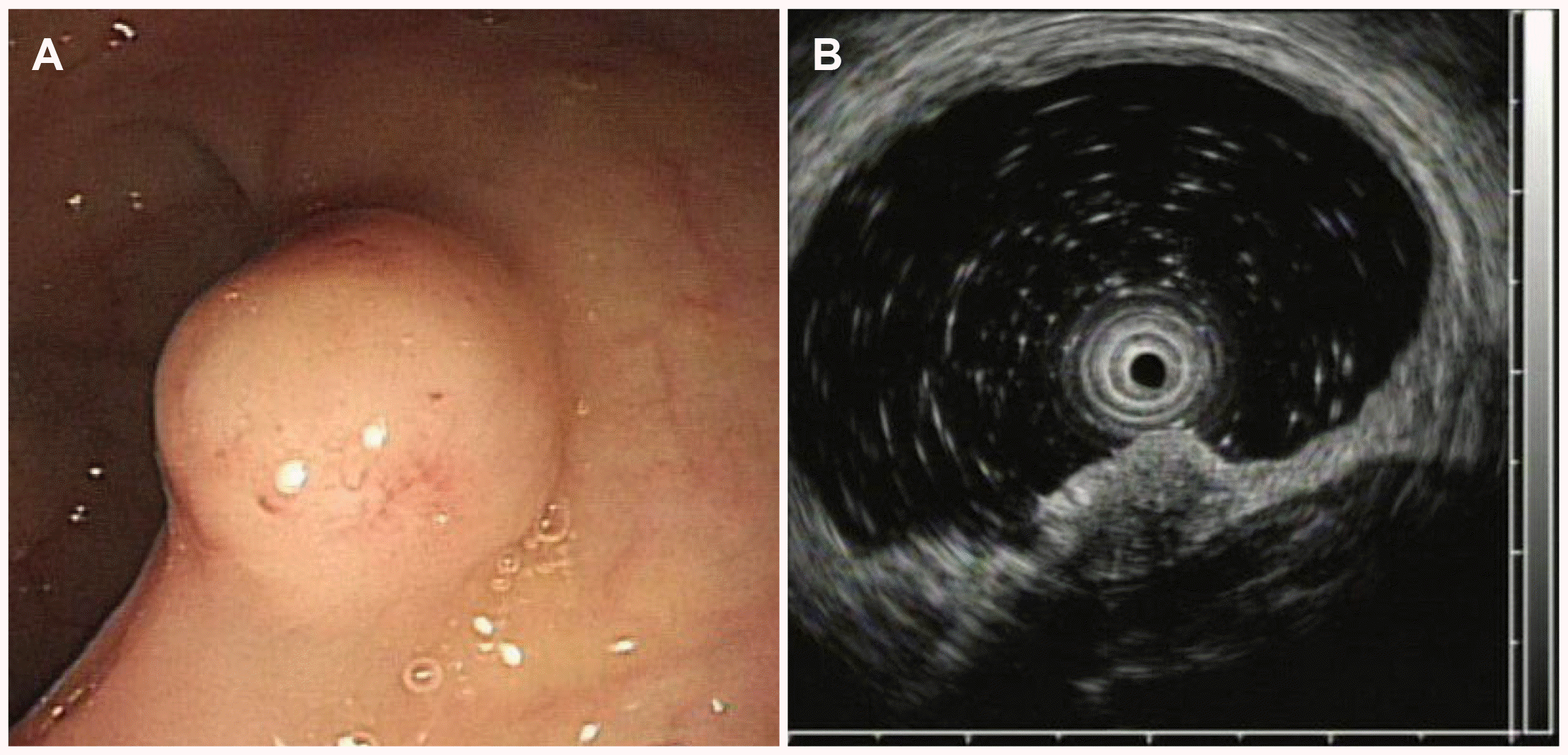
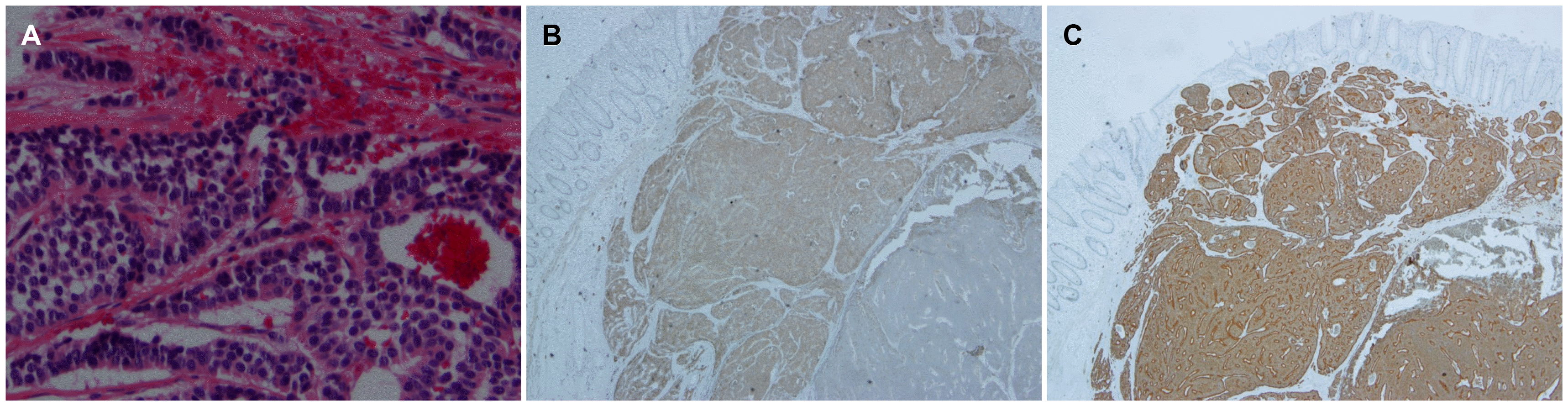
A 65-year-old man visited the St. Vincent's Hospital for a further evaluation of a rectal subepithelial tumor detected on routine screening colonoscopy. He had been taking medications for diabetes and hypertension but had no other specific medical or family history. The patient was never a smoker and only a social drinker. He had no complaints of symptoms, such as abdominal pain or changes in bowel habits. At the first visit, there were no abnormalities in the laboratory investigations, which included a complete blood count, blood chemistry, and serum carcinoembryonic antigen. Colonoscopy revealed an ovoid, yellowish protruding lesion, 8 mm in size, with normal overlying mucosa in the mid rectum (Fig. 1A). Upon an endoscopic ultrasonographic examination, a poorly demarcated, hypoechoic mass lesion was observed in the submucosal layer (Fig. 1B). Under the suspicion of NET, an endoscopic mucosal resection (EMR) was performed. During the procedure, normal saline was injected into the submucosa to lift the lesion, which had been resected completely using an electrical snare. Histological analysis revealed many round cells that contained similar-size nuclei and formed nests or cord-like structures. The lateral and vertical resection margins were negative for tumors, and no local muscle layer infiltration and lymphovascular invasions were observed. Immunohistochemical staining revealed CD 56a and synaptophysin positivity in most of the cells (Fig. 2). The mitotic number of the specimen was only 1 in 10 high-power fields. The Ki-67 index was 0.76%. Collectively, these data led to a diagnosis of grade 1 (G1) rectal NET, and an en-bloc resection was performed. Abdominal CT performed immediately after EMR showed no evidence of lymph node involvement or distant metastasis.
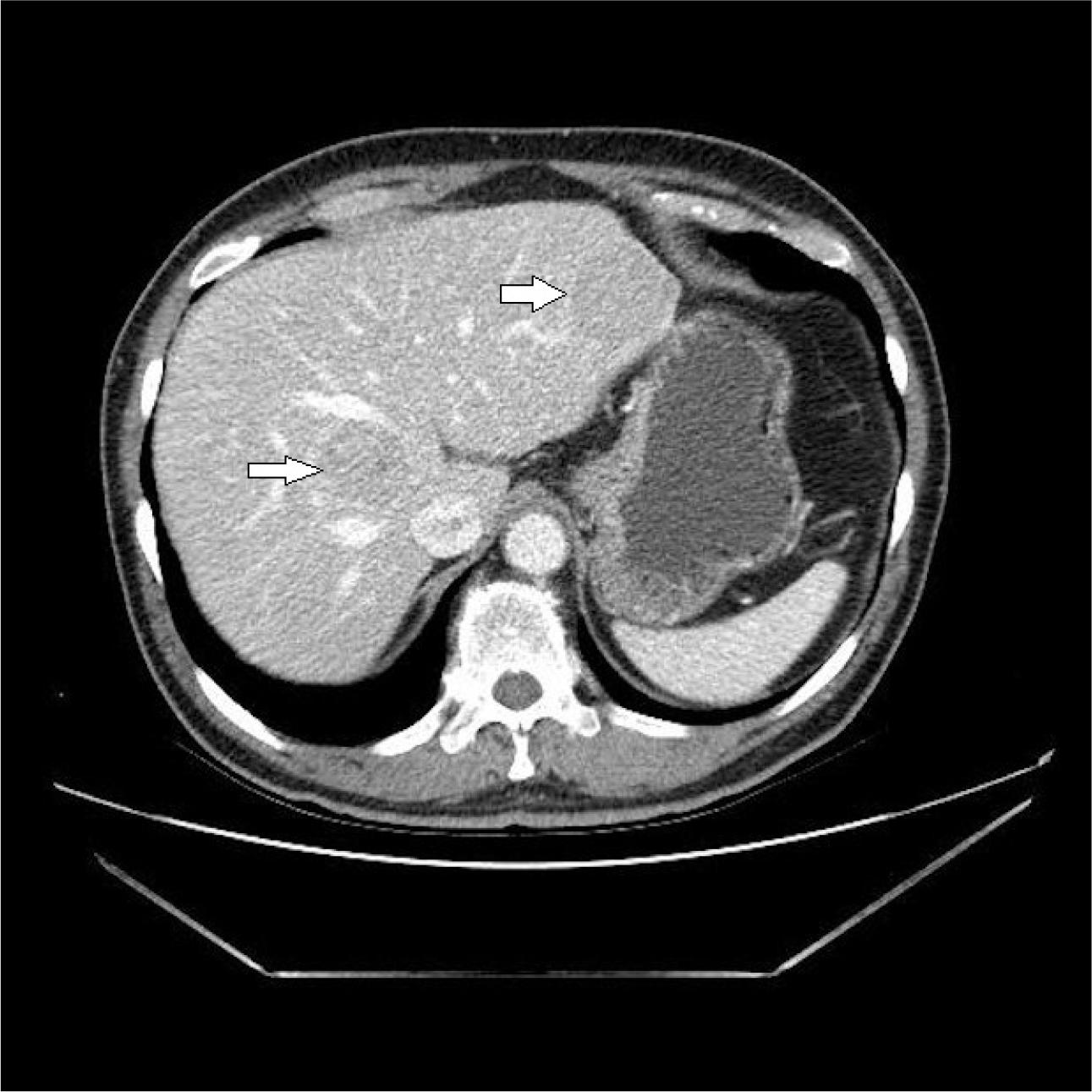
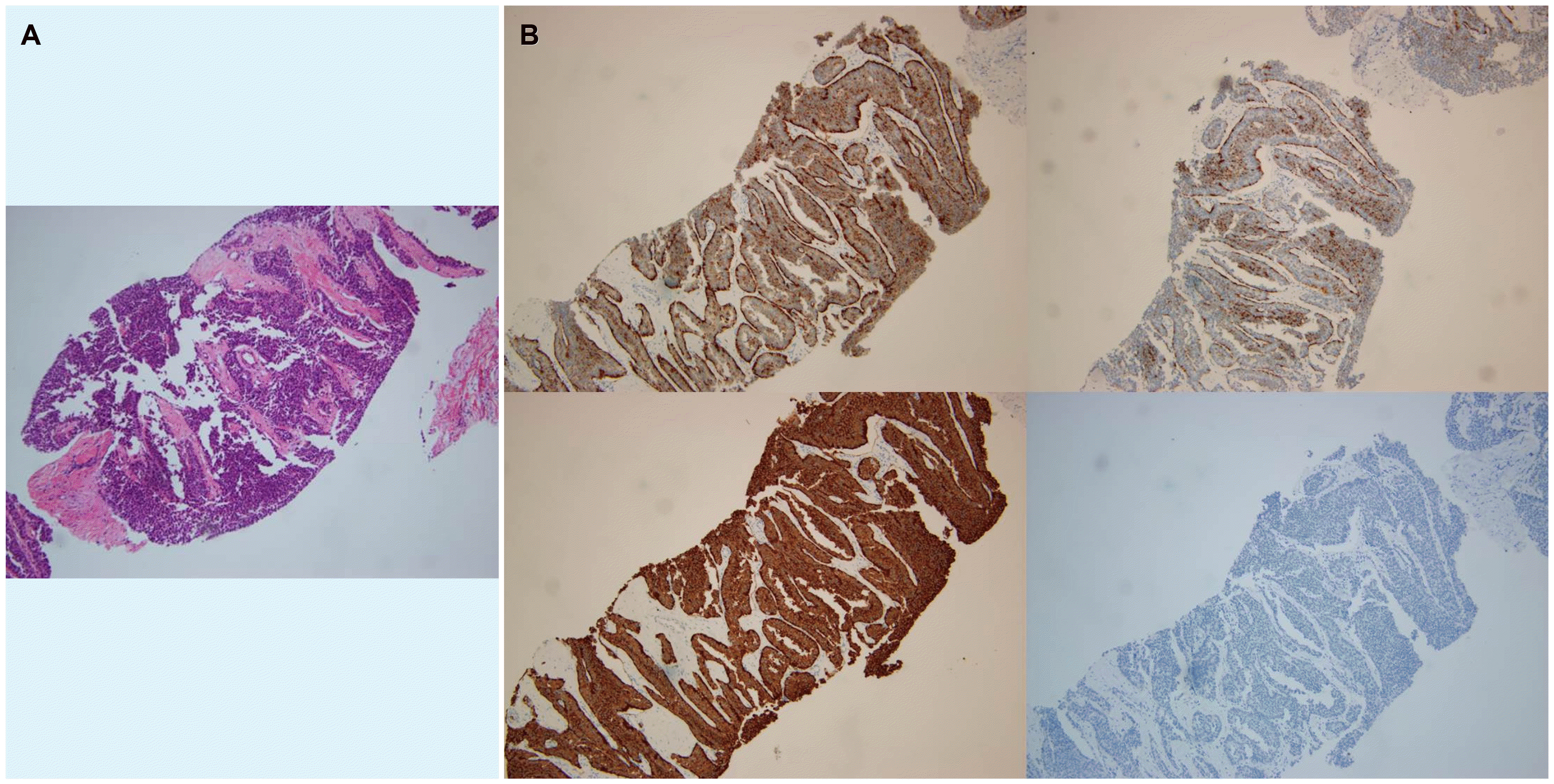
A follow-up colonoscopy was scheduled one year later, but the patient did not visit the clinic until 5 years after the EMR procedure. The patient re-visited for a check-up for general conditions, and colonoscopy and abdominal CT were performed. Colonoscopy did not show any sign of local recurrence, but CT revealed multiple liver masses of varying sizes with hyper-attenuated enhancement, which were not observed previously (Fig 3). An ultrasound-guided liver biopsy was performed. Histopathological analysis revealed nest-forming, regular-shaped cells that were positive for CD56a, synaptophysin, and chromogranin (Fig. 4). The hepatic masses were diagnosed as NET-origin metastatic lesions, and the tumors were classified as high grade based on the high mitotic number (9 in 10 high-power fields) and Ki-67 index (30%). Subsequent chest CT and PET CT failed to determine the other focus of the hepatic metastasis. Furthermore, PET CT revealed suspicious nodular foci for metastatic lymph nodes were identified in the right perirectal area. Based on the above, the rectal NET, which had already been removed completely, recurred in the form of multiple liver metastases. Attempts were made to design a treatment strategy, but the patient visited another medical center to seek a second opinion.
Go to : 
NETs can appear anywhere in the body, but most occur in the gastrointestinal tract, pancreas, and bronchial system.5,6 They are relatively rare among gastrointestinal polypoid lesions; those with good differentiation are found primarily in the rectum. NETs can exhibit various malignant processes depending on microscopic findings, even if the differentiation is good. In this context, classification based on the histopathological feature(s) better reflects the nature of the disease rather than the term “carcinoid tumor”, which has been used in the past.7
In 2010, the system proposed by the World Health Organization classified rectal NETs based on Ki-67 cell proliferation and the mitosis count indices to reflect the proliferative capacity of the tumors.2,7 According to a recent systematic review, 79% of tumors had less than 1 cm in size, and 89% of the lesions were low-grade tumors that were limited to the submucosal layer. The prevalence of concomitant lymph node metastasis and distant metastasis was 8% and 4%, respectively.4
Rectal NETs have a more favorable prognosis than adenocarcinoma, for which the 5-year survival rate has been reported to be 75.2-88.3%.4 The disease stage has the most significant effect on the prognosis. If any lymph node or distant metastasis has occurred, there will be noticeable declines in the 5-year survival. The risk factors for regional or distant metastases include tumor size, involvement of the muscularis propria, differentiation index, and vascular/neural involvement.2,4,8 In particular, the prevalence of metastasis varies greatly, from 1.7% to 50%, depending on whether the lesion is <1 cm or larger >2 cm.9
In the case of the present patient, a 0.8 cm sized NET was found in the mid rectum, with no evidence of lymph node involvement or distant metastasis on abdominal CT imaging at the time of the endoscopic procedure. Vertical and lateral margins of the resected specimen were tumor-free, and the histological grade was reported to be G1. According to the guidelines for the treatment and surveillance of rectal NETs from the National Comprehensive Cancer Network, small lesions <1 cm in size do not need to be monitored unless the resection margins are involved.10 On the other hand, some studies showed results that were contrary to the above guidelines. A recent study using a hospital‐based registry reported that even rectal NETs, less than 1 cm in size, showed lymph node positivity in 11% of patients.11 A retrospective study of US-categorized patients of rectal NETs with a distant metastasis by the lesion size and histopathologic grades and reported that diminutive rectal NETs (<1 cm in size) could induce a distant metastasis, but they were all included in Grade 2 or 3 tumors.12 Similar to the present case, several studies have reported NETs with lymph node invasion or distant metastases, even if the original lesions themselves were low-grade or small in size.13-16 Based on these examples, regardless of how small the low-grade NET is, it is difficult to exclude the possibility of hidden metastasis completely. Therefore, the controversy regarding whether periodic follow-up is necessary is likely to persist.
This case was distinct compared to previous reports in that the patient was in the state of ‘cured’. The small, G1 NET lesion was resected completely by EMR, and there was no evidence of residual disease. On the other hand, the disease recurred 5 years later with multiple liver metastases, which was unpredictable despite closely following the current guidelines. Although the possibility of primary hepatic NETs needs to be considered, the hepatic lesions were concluded to be recurring rectal NET because a primary hepatic NET is very rare,17 and metastatic lymph nodes were found in the perirectal area by PET CT.
Rectal NETs are rare diseases that can be encountered during a routine colonoscopy. Generally, most NETs have a benign natural course, particularly small lesions (<10 mm) with a low histopathological grade exhibit a satisfactory prognosis after an endoscopic resection. The current guidelines state that follow-up examinations are unnecessary when small NETs are resected completely. On the other hand, aggressive, rapidly progressive NETs may be unexpectedly present in practice. Therefore, in planning management strategies, it is also helpful to be aware of the possibility of undesirable situations rather than strictly adhering to the general guidelines.
Go to : 
REFERENCES
1. McDermott FD, Heeney A, Courtney D, Mohan H, Winter D. 2014; Rectal carcinoids: a systematic review. Surg Endosc. 28:2020–2026. DOI: 10.1007/s00464-014-3430-0. PMID: 24584484.

2. Weinstock B, Ward SC, Harpaz N, Warner RR, Itzkowitz S, Kim MK. 2013; Clinical and prognostic features of rectal neuroendocrine tumors. Neuroendocrinology. 98:180–187. DOI: 10.1159/000355612. PMID: 24080744.

3. Anthony LB, Strosberg JR, Klimstra DS, et al. 2010; The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (nets): well-differentiated nets of the distal colon and rectum. Pancreas. 39:767–774. DOI: 10.1097/MPA.0b013e3181ec1261. PMID: 20664474.
4. Rodrigues Â, Castro-Poças F, Pedroto I. 2015; Neuroendocrine rectal tumors: main features and management. GE Port J Gastroenterol. 22:213–220. DOI: 10.1016/j.jpge.2015.04.008. PMID: 28868410. PMCID: PMC5579972.

5. Modlin IM, Oberg K, Chung DC, et al. 2008; Gastroenteropancreatic neuroendocrine tumours. Lancet Oncol. 9:61–72. DOI: 10.1016/S1470-2045(07)70410-2. PMID: 18177818.

6. Maggard MA, O'Connell JB, Ko CY. 2004; Updated population-based review of carcinoid tumors. Ann Surg. 240:117–122. DOI: 10.1097/01.sla.0000129342.67174.67. PMID: 15213627. PMCID: PMC1356383.

7. Bosman FT, Carneiro F, Hruban RH, Theise ND. 2010. WHO classification of tumours of the digestive system. 4th ed. International Agency for Research on Cancer;Lyon:
8. Chablaney S, Zator ZA, Kumta NA. 2017; Diagnosis and management of rectal neuroendocrine tumors. Clin Endosc. 50:530–536. DOI: 10.5946/ce.2017.134. PMID: 29207857. PMCID: PMC5719921.

9. Yoon SN, Yu CS, Shin US, Kim CW, Lim SB, Kim JC. 2010; Clinicopathological characteristics of rectal carcinoids. Int J Colorectal Dis. 25:1087–1092. DOI: 10.1007/s00384-010-0949-y. PMID: 20397020.

10. Neuroendocrine and adrenal tumors. 2019. Jan. 7. [Internet]. NCCN;Plymouth Meeting (PA): Available from: https://www.nccn.org/professionals/physician_gls/pdf/neuroendocrine.pdf. cited 2020 Jan 5.
11. Gamboa AC, Liu Y, Lee RM, et al. 2019; A novel preoperative risk score to predict lymph node positivity for rectal neuroendocrine tumors: an NCDB analysis to guide operative technique. J Surg Oncol. 120:932–939. DOI: 10.1002/jso.25679. PMID: 31448820. PMCID: PMC6791747.

12. Folkert IW, Sinnamon AJ, Concors SJ, et al. 2020; Grade is a dominant risk factor for metastasis in patients with rectal neuroendocrine tumors. Ann Surg Oncol. 27:855–863. DOI: 10.1245/s10434-019-07848-0. PMID: 31701298.

13. Hirose Y, Sakata J, Endo K, et al. 2018; A 0.8-cm clear cell neuroendocrine tumor G1 of the gallbladder with lymph node metastasis: a case report. World J Surg Oncol. 16:150. DOI: 10.1186/s12957-018-1454-y. PMID: 30037336. PMCID: PMC6057040.

14. Matsuhashi N, Takahashi T, Tomita H, et al. 2017; Evaluation of treatment for rectal neuroendocrine tumors sized under 20 mm in comparison with the WHO 2010 guidelines. Mol Clin Oncol. 7:476–480. DOI: 10.3892/mco.2017.1326. PMID: 28894583. PMCID: PMC5582451.

15. Nagata K, Tajiri K, Shimada S, et al. 2017; Rectal neuroendocrine tumor G1 with a solitary hepatic metastatic lesion. Intern Med. 56:289–293. DOI: 10.2169/internalmedicine.56.7523. PMID: 28154272. PMCID: PMC5348452.

16. Atwal D, Joshi KP, Jeffus S, Ntambi J, Mahmoud F. 2017; Well-differentiated neuroendocrine tumor-A low B-grade tumor's aggressive course and dismal outcome: a case report. Perm J. 21:16–193. DOI: 10.7812/TPP/16-193. PMID: 28633725. PMCID: PMC5478591.

17. Song JE, Kim BS, Lee CH. 2016; Primary hepatic neuroendocrine tumor: a case report and literature review. World J Clin Cases. 4:243–247. DOI: 10.12998/wjcc.v4.i8.243. PMID: 27574614. PMCID: PMC4983697.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download