This article has been
cited by other articles in ScienceCentral.
Abstract
ERCP is the standard treatment for common bile duct stones. On the other hand, 10-15% of cases involve intractable common bile duct stones, which cannot be treated by conventional biliary sphincterotomy with a stone retrieval method. Large bile duct stones are typically managed by mechanical lithotripsy and endoscopic papillary large balloon dilatation. Peroral cholangioscopy techniques can be applied if this technique fails. In the present case, a 67-year-old woman had a large common bile duct stone that could not be retracted using the conventional ERCP stone extraction method. The common bile duct stone was eventually removed by direct peroral upper gastrointestinal endoscopy and a polypectomy snare.
Go to :

Keywords: Choledocholithiasis, Cholangiopancreatography, endoscopic retrograde, Endoscopy, gastrointestinal, therapeutic use, Lithotripsy
INTRODUCTION
Common bile duct (CBD) stones are not rare. The prevalence of CBD stones is estimated to be 10-20% among gall-stone patients. Moreover, approximately 10% of patients who undergo cholecystectomy have CBD stones.
1,2 A CBD stone can provoke fatal complications, such as obstructive jaundice, acute pyogenic cholangitis, acute pancreatitis, liver abscess, and secondary biliary cirrhosis.
The gold standard treatment for CBD stones is ERCP. Most bile duct stones can be retracted using standard ERCP techniques, such as endoscopic sphincterotomy (EST) or extraction balloon retrieval. Nevertheless, the success rate of stone extraction after EST is approximately 85-90%, even for experts in pancreatobiliary endoscopy.
3 Approximately 10-15% of intractable CBD stone cases are difficult to extract.
4 Among the intractable CBD stones, large stones are typically managed by mechanical lithotripsy and endoscopic papillary large balloon dilatation (EPLBD). If these techniques fail, a range of cholangioscopy techniques can be applied.
Recently, a combination of peroral cholangioscopy (POCS) and either electrohydraulic lithotripsy (EHL) or laser lithotripsy (LL) is being used more often for the treatment of large bile duct stones for fragmentation. Mother-baby cholangioscopy, SpyGlass, and ultra-slim endoscopy are used for POCS, but there are no reports of upper gastrointestinal endoscopy for direct POCS. This paper reports the first case of the successful removal of a large, intractable CBD stone by direct peroral upper gastrointestinal endoscopy and a polypectomy snare.
Go to :

CASE REPORT
A 67-year-old woman visited the emergency department of Kyungpook National University Hospital with the chief complaint of a fever. She also complained of anorexia for one month accompanied by an intermittent febrile sense, epigastric pain, and headache. Her vital signs were as follows: blood pressure 101/74 mmHg, pulse rate 82/min, respiratory rate 20/min, and body temperature of 38.8℃. Four years ago, she had undergone ERCP 3 times because of a large CBD stone, 54×32 mm in size. On the other hand, the stone could not be removed despite EST, extraction balloon/basket retrieval, and EPLBD. An endoscopic biliary plastic stent (plastic, double pigtail, 7 Fr×100 mm, AdvanixTM Biliary Stent, Boston Scientific, Marlborough, MA, USA) was inserted, and she was followed up in the outpatient department. The biliary plastic stent migrated 3 years ago, but she had no symptoms until this case.
The laboratory findings were as follows: white blood cell count 22,680/µL (neutrophil 95.1%), hemoglobin 10.0 g/dL, platelet count 392,000/µL, aspartic acid aminotransferase 104 IU/L, alanine aminotransferase 91 IU/L, alkaline phosphatase 260, gamma-glutamyl transferase 161, blood urea nitrogen 5.7 mg/dL, creatinine 0.84 mg/dL, and CRP 10.48 mg/dL. A large CBD, 54×32 mm in size, was observed with multiple intrahepatic duct stones in the abdominal computed tomography scan conducted on the admission day. The stone size had not changed when compared with the image taken 4 years ago. A suspected pericholangitis abscess, 5 cm in size, was also observed in liver segment 4 (
Fig. 1). Percutaneous transhepatic biliary drainage (PTBD) and percutaneous drainage for abscess were conducted on the same admission day. On the 3rd day of hospitalization, an attempt was made to remove the CBD stone by PTBD, but the stone was too large to be caught in the snare. A wire loop technique using a snare was then attempted, but again, the stone could not be caught in the basket, despite several trials.
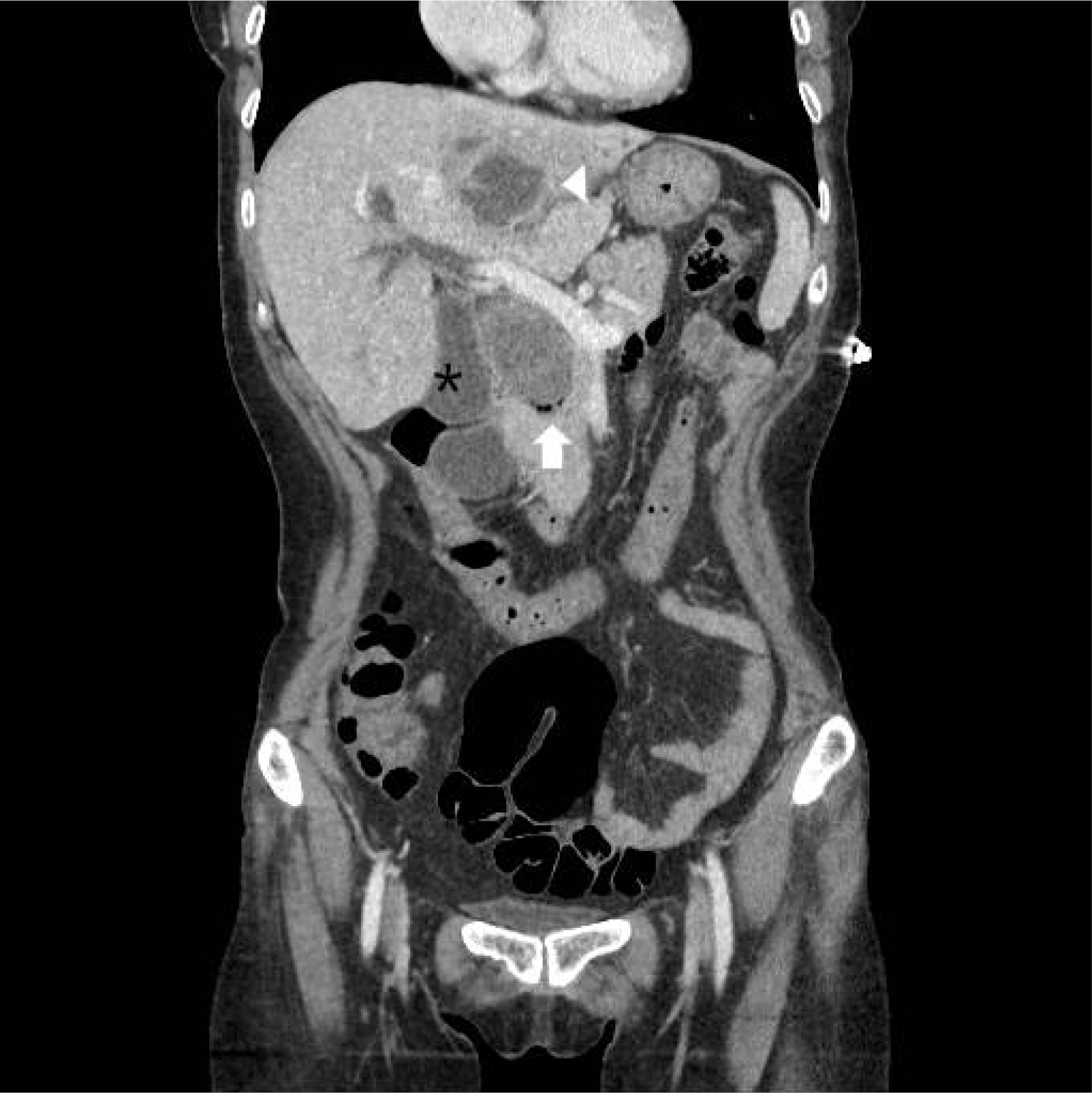 | Fig. 1Abdominal computed tomography on the admission day. The intrahepatic duct, common hepatic duct, and common bile duct (CBD) were dilated. The large CBD stone, size 54×32 mm, was observed in the CBD (arrow). A cystic lesion with peripheral rim enhancement, 50 mm in size, was observed in liver segment 3 (arrowhead). The gallbladder (asterisk) was dilated. 
|
On the 5th day of hospitalization, ERCP again was performed, but the stone could not be caught in the basket. Retrieval balloon extraction did not move the stone from the bile duct out to the papilla. EPLBD (CRE
TM balloon, Boston Scientific, 12-15 mm size, eight atmospheres pressure, 3 minutes) was used again. Finally, upper gastrointestinal endoscopy (GIF H260, Olympus, Tokyo, Japan) was used as direct POCS with the polypectomy snare (Large Oval-Medium Stiff, loop with 30 mm, minimum working cannel 2.8 mm, Boston Scientific). After inserting the upper gastrointestinal endoscope into the CBD, a brown pigment large bile duct stone could be observed in the CBD (
Fig. 2A), and the CBD stone was then captured with a snare during which the stone cracked as a result of the powerful mechanical grasp by the snare (
Fig. 2B). While cracking the stone using polypectomy snare, some fragments were extracted by the snare (
Fig. 2C, D). After additional snaring 4-5 times, the large CBD stone was cracked down into small pieces (
Fig. 2E). Finally, whole fragmented stones were removed with a retrieval balloon and basket. The procedure time for snare fragmentation and stone removal was approximately 30 minutes. On the 10th day of admission, a follow-up chol-angiogram by PTBD was conducted. The PTBD and percutaneous drainage were removed after confirming the clear up of the CBD (
Fig. 3). She was discharged on the 11th day from admission and has been followed up without complaint in the outpatient department.
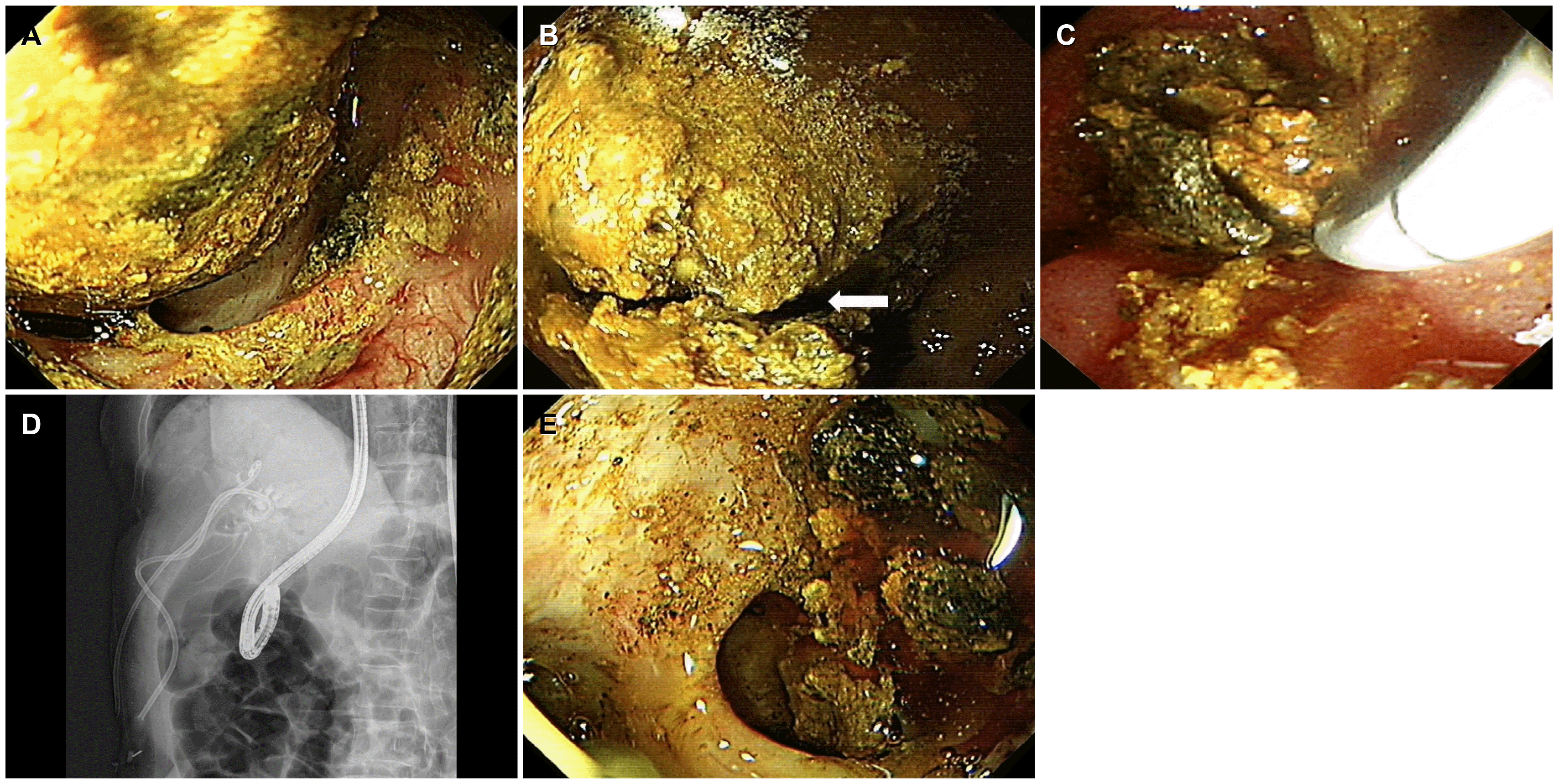 | Fig. 2Removal of common bile duct (CBD) stone by direct peroral cholangioscopy using upper gastrointestinal endoscopy and polypectomy snare. (A) A huge CBD stone showed brown pigment characteristics. The CBD was dilated significantly by the stone. (B) The CBD stone had been cracked into two pieces by grasp with snare polypectomy at the midline of the stone (arrow). (C) During cracking the stone by polypectomy snare, some fragmentations were extracted by the snare. (D) The upper gastrointestinal endoscope with snare was inserted inside the CBD.(E) After several attempts of the polypectomy snare technique, the stone was fragmented into several small pieces, which were extracted by basket and retrieval balloon. 
|
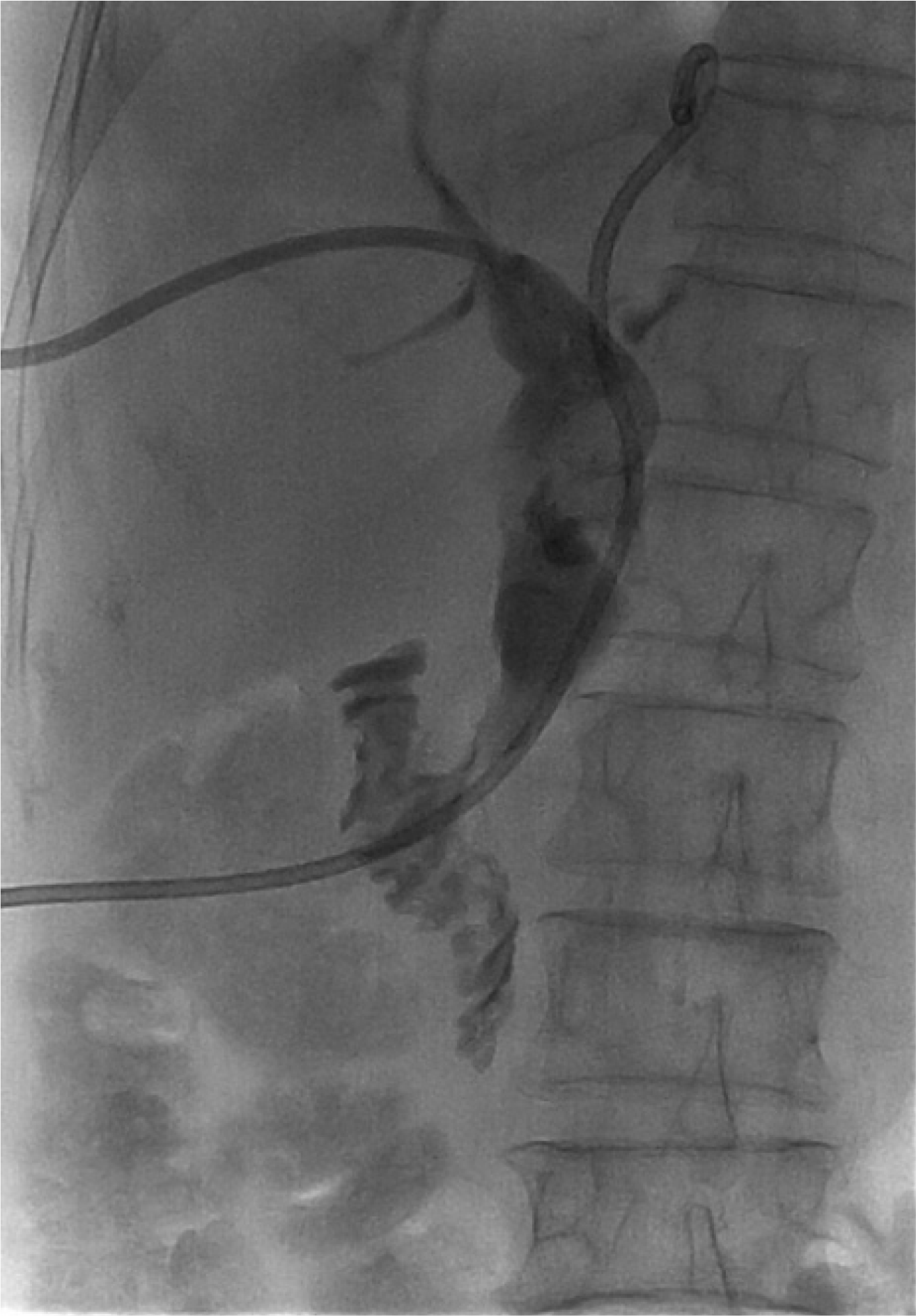 | Fig. 3Follow-up cholangiogram by percutaneous transhepatic cholangioscopy before removal showed no remnant common bile duct stone. 
|
Go to :

DISCUSSION
Difficult bile duct stones can be classified according to the anatomy, characteristics of the stones, location, and patient’s condition.
5 Among the characteristics of stones, stones larger than 15 mm are difficult to extract.
6 Any definition of a large CBD stone should include a relation to the lower CBD diameter to enable a relative comparison. Doshi et al. proposed the definition of a large CBD stone as >15 mm with a stone to CBD ratio of >1.0.
7
Several ERCP techniques for treatment, such as mechanical lithotripsy, EPLBD, biliary stenting, and extracorporeal shock-wave lithotripsy, can be used alone or in combination for a large CBD stone. On the other hand, large stones with a diameter ≥20 mm usually require fragmentation before extraction.
7 In particular, huge CBD stones ≥30 mm cannot usually be captured by a basket for mechanical lithotripsy. In such cases, POCS can be attempted by either one of three endoscopy methods: 1) dual operator cholangioscopy (mother-baby scope), 2) single-operator cholangioscopy (SpyGlass Direct Visualization DS Boston Scientific), and 3) direct cholangioscopy with an ultra-slim endoscope. POCS can be used to fragment the stone with EHL/LL. The feasibility and efficacy of direct POCS using an ultra-slim upper endoscope have also been validated. EHL or LL under direct POCS has been reported to be successful in 85-89% of cases of difficult stones.
8,9 On the other hand, these fragmentation techniques are still complicated and expensive. There are also concerns regarding the injury risks of the POCS lens when EHL or LL is used. Moreover, in many hospitals, the electroprobe and generator devices for EHL or LL usually belong to the urology department.
A polypectomy snare was used to fragment the CBD stone in our case. This method is already used in bezoar treatment. After injecting Coca-Cola, the snare polypectomy is used for fragmentation when the bezoar is ready to be fragmented with friability.
10 In the present case, the stone was large, but the morphology did not look too difficult for fragmentation when the stone was observed by direct cholangioscopy with upper gastrointestinal endoscopy. Primary CBD stones are prominent in East Asia. CBD stones are usually brown-pigmented stones that form because of bile stasis and infections.
11 Therefore, fragmentation with a snare could be successful. In addition, unlike snaring by PTBD, direct cholangioscopy enables observations of the stone in an endoscopic view rather than in a fluoroscopic view. Hence, the midline of the stone could be captured for fragmentation.
Adverse effects of cholangioscopy have been reported.
12 Although a rare complication, air embolism is a potentially fatal complication of cholangioscopy.
13 To prevent air embolism, carbon dioxide (CO
2) or water irrigation is strongly recommended during cholangioscopy. In the present case, however, cholangioscopy was performed under room air condition. Although upper GI endoscopy was inserted carefully with minimal air insufflation, there was an apparent risk of an air embolism. Recently, there was a report describing fatal cases of CO
2 emboli during cholangioscopy.
14 The cholangioscopy procedure should be performed under a well-controlled CO
2 regulator or water irrigation.
In general, the polypectomy snare cannot be inserted into the ultra-slim endoscopy. The working channel of ultra-slim endoscopy (diameter 2.0-2.2 mm) is too small to advance the conventional polypectomy snare (minimum working cannel 2.8 mm). Therefore, when using the snare technique to remove a large CBD stone, prior CBD dilatation is essential for the insertion of upper gastrointestinal endoscopy, which has a large working channel. The balloon dilatation should be made up to the CBD stone diameter for conventional stone removal. If the CBD stone diameter is larger than 30 mm, the insertion of upper gastrointestinal endoscopy is possible as the bile duct dilatation should be more than 30 mm. In addition, a small diameter micro or mini snare should be developed for ultra-slim POCS or SpyGlass cholangioscopy in the near future.
A large CBD stone over 30 mm is difficult to remove. The CBD stone was removed successfully by direct POCS using an upper endoscope and polypectomy snare. In cases where cholangioscopy with EHL or LL cannot be used, this technique can be considered for intractable large CBD stones. In addition, a well-controlled CO2 regulator or water irrigation is strongly recommended during cholangioscopy to prevent air embolisms.
Go to :







 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download