An 88-year-old woman was admitted to Chung-Ang University Hospital due to melena. She had a prior medical history of hypertension and chronic kidney disease. The peripheral blood cell counts showed anemia with a hemoglobin level of 3.8 g/dL. Esophagogastroduodenoscopy (EGD) was performed to determine the source of suspected upper GI bleeding. The endoscopic examination revealed a 2-cm sized, irregular-shaped ulcerofungating mass on the greater curvature, high body of the stomach (
Fig. 1A). An endoscopic biopsy was performed to obtain a pathologic diagnosis of GIST. Abdominal CT revealed an 8.9 cm sized exophytic growing, heterogenous enhancing mass adjacent to the gastric high body (
Fig. 1B). There was no evidence of a distant metastasis. She had undergone a laparoscopic wedge resection of the stomach for the gastric GIST. A pathology evaluation confirmed that the lesion was GIST with a high risk of progressive disease. The tumor size was 9.0×6.0×5.3 cm, and the mitotic index was a 16/50 HPF. The histologic type was the mixed spindle and epithelioid type (
Fig. 2A). Immunohistochemistry confirmed GIST with diffuse positive staining for CD117, CD34, and DOG1, with a low Ki-67 proliferation index (
Fig. 2B-E). There was no involvement of the tumor at the resection margin on the microscopic evaluation. After surgery, she received an adjuvant imatinib therapy at an initial dose of 400 mg/day to prevent a recurrence of the disease. On the other hand, recurrent anemia occurred during treatment, and the dose of imatinib was reduced. Despite the dose reduction, however, the severe anemia persisted, and the hemoglobin level decreased to 4.6 g/dL. Imatinib therapy was discontinued after 18 months of maintenance owing to severe drug intolerance. She had undergone EGD 1 year after the surgery and abdominal CT every 6 months, which showed no signs of tumor recurrence. She underwent a follow-up EGD 40 months after surgery, which revealed a 2-cm-sized round-shaped mass at the anastomosis site (
Fig. 3A). Subsequent abdominal CT was performed as local recurrence was suspected (
Fig. 3B). A laparoscopic wedge resection of the gastric mass was performed because there was no sign of distant metastasis. The histology was a spindle cell type GIST with high risk, showing a tumor size of 2.6×2.3×2.0 cm (
Fig. 4A). The mitotic index was more than 10/50 HPF, with a high Ki-67 proliferation index of 30% (
Fig. 4B). The resection margin was free from tumor involvement. After 2 months of adjuvant imatinib therapy at the lower dose (300 mg/day), severe anemia occurred again, which led to discontinuation of the drug. Eighteen months after the second surgery, a newly appeared 2.6 cm mass was detected on abdominal CT, and subsequent EGD revealed a mass located at the anastomosis line (
Fig. 5A). Considering her very old age and poor performance status, she was followed up without treatment. Six months later, an abdominal CT scan showed progressive disease with invasion to the adjacent organs and multiple intra-abdominal metastases (
Fig. 5B). She is now on intermittent imatinib therapy with a reduced dose (200 mg/day) due to her old age and the drug intolerance, while carefully monitoring the hemoglobin count. Follow-up CT taken after 2 months of medication revealed a partial response of the recurred disease, and she is currently being treated in an outpatient clinic setting.
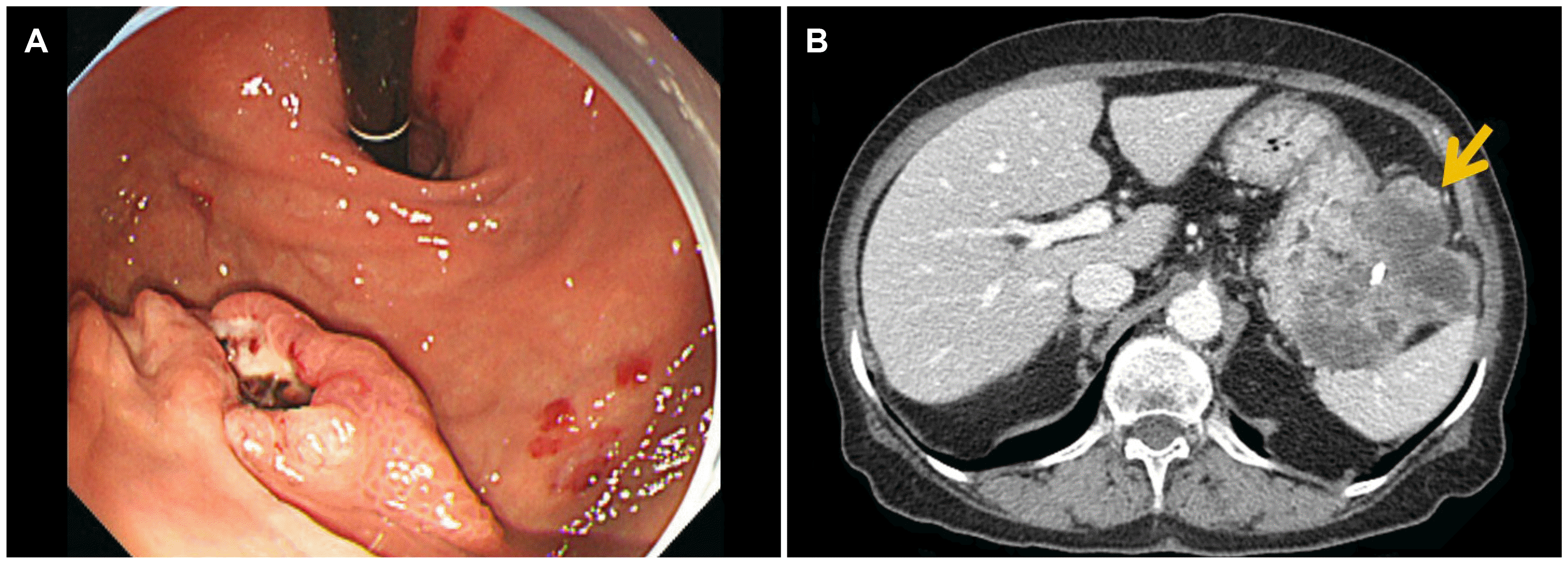 | Fig. 1Endoscopic images of the gastric lesion and abdominal computed tomography findings at the initial presentation. (A) A 2-cm sized, irregular shaped ulcerofungating mass on the greater curvature of the high body of the stomach. (B) An exophytic, heterogeneous enhancing mass with necrosis and central calcification, located adjacent to the gastric high body, closely abutting the splenic hilum (arrow). 
|
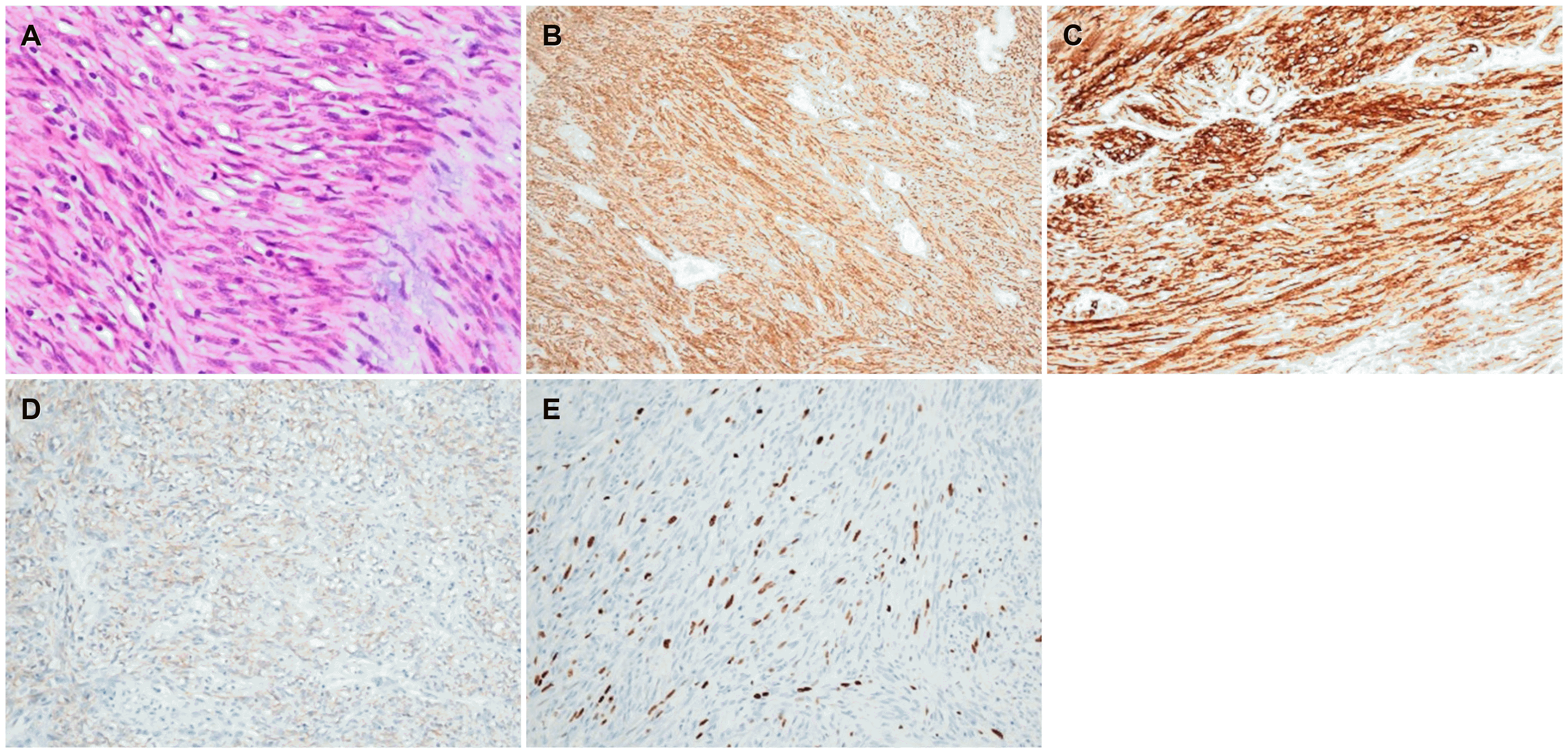 | Fig. 2Histologic features of the gastric gastrointestinal stromal tumor at the first surgery. (A) Histopathological evaluation reveals blandly spindle cells with faintly eosinophilic cytoplasm in a syncytial pattern. Elongated nuclei with inconspicuous nucleoli are noted (H&E, ×400).(B) Diffuse positive cytoplasmic staining for CD117 (Immunohistochemical staining, ×20). (C) Diffuse positive staining for CD34 (Immunohistochemical staining, ×20). (D) Focal positive cytoplasmic staining for DOG-1 (Immunohistochemical staining, ×20). (E) Ki-67 proliferation index is less than 5% (Immunohistochemical staining, ×20). 
|
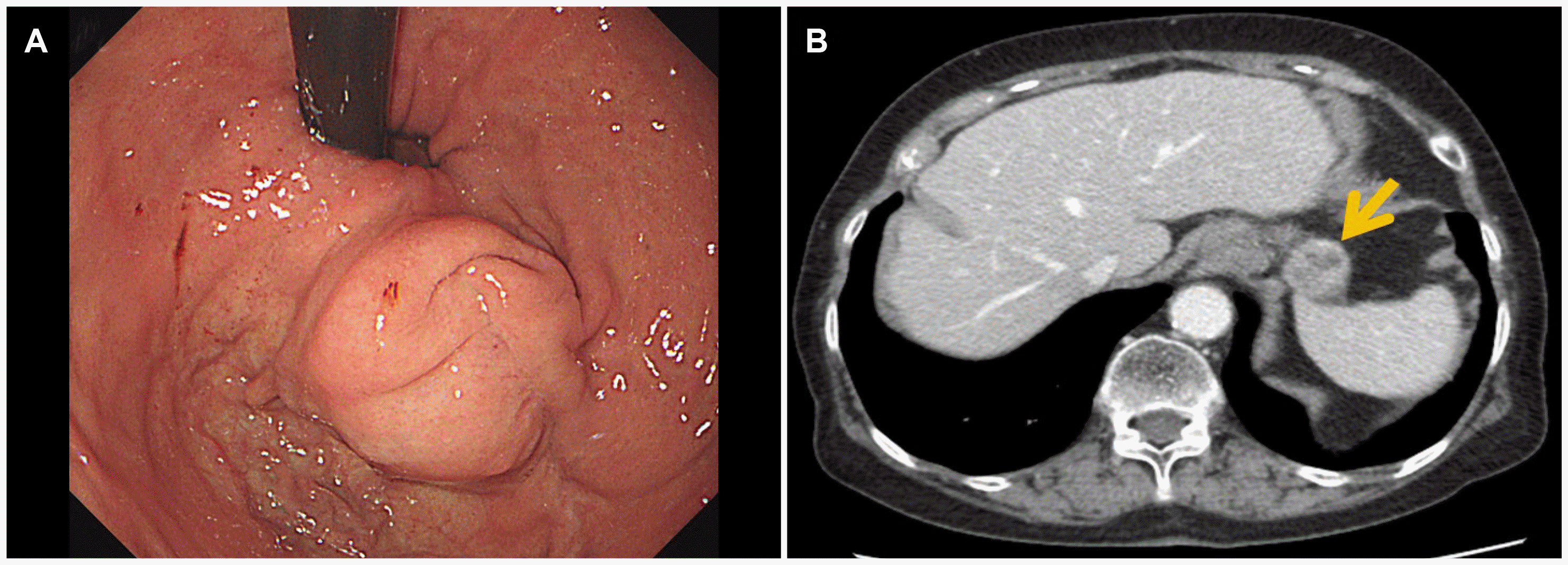 | Fig. 3Endoscopic images of the recurred gastric lesion and abdominal computed tomography findings at the first recurrence. (A) A 2-cm sized spherical shaped subepithelial mass lying on the anastomosis site. (B) An exophytic, heterogeneously enhancing mass at the anastomosis site of the stomach, measuring 2.6 cm, without signs of extragastric metastasis (arrow). 
|
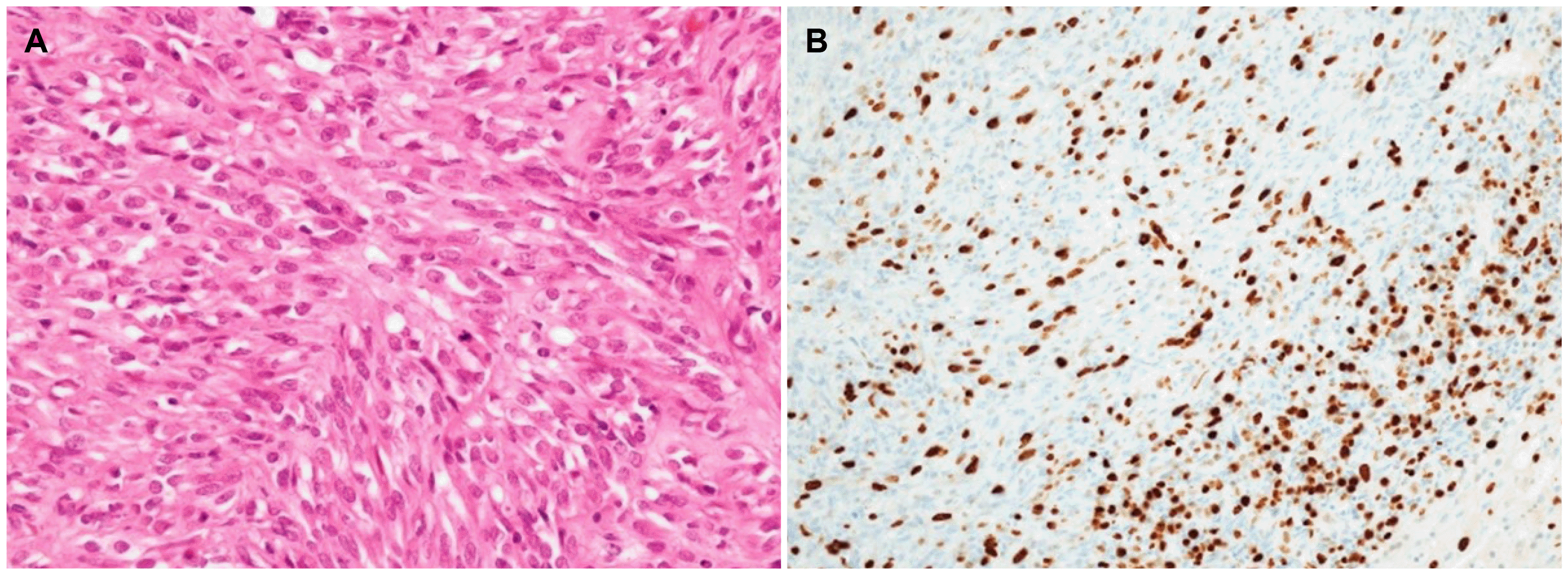 | Fig. 4Histologic features of a gastric gastrointestinal stromal tumor at the second surgery. (A) Histopathological evaluation revealed spindle cell proliferation with frequent mitoses up to 159/50 high-power fields (H&E, ×40) (B) Ki-67 proliferation index is 30% (Immunohistochemical staining, ×20). 
|
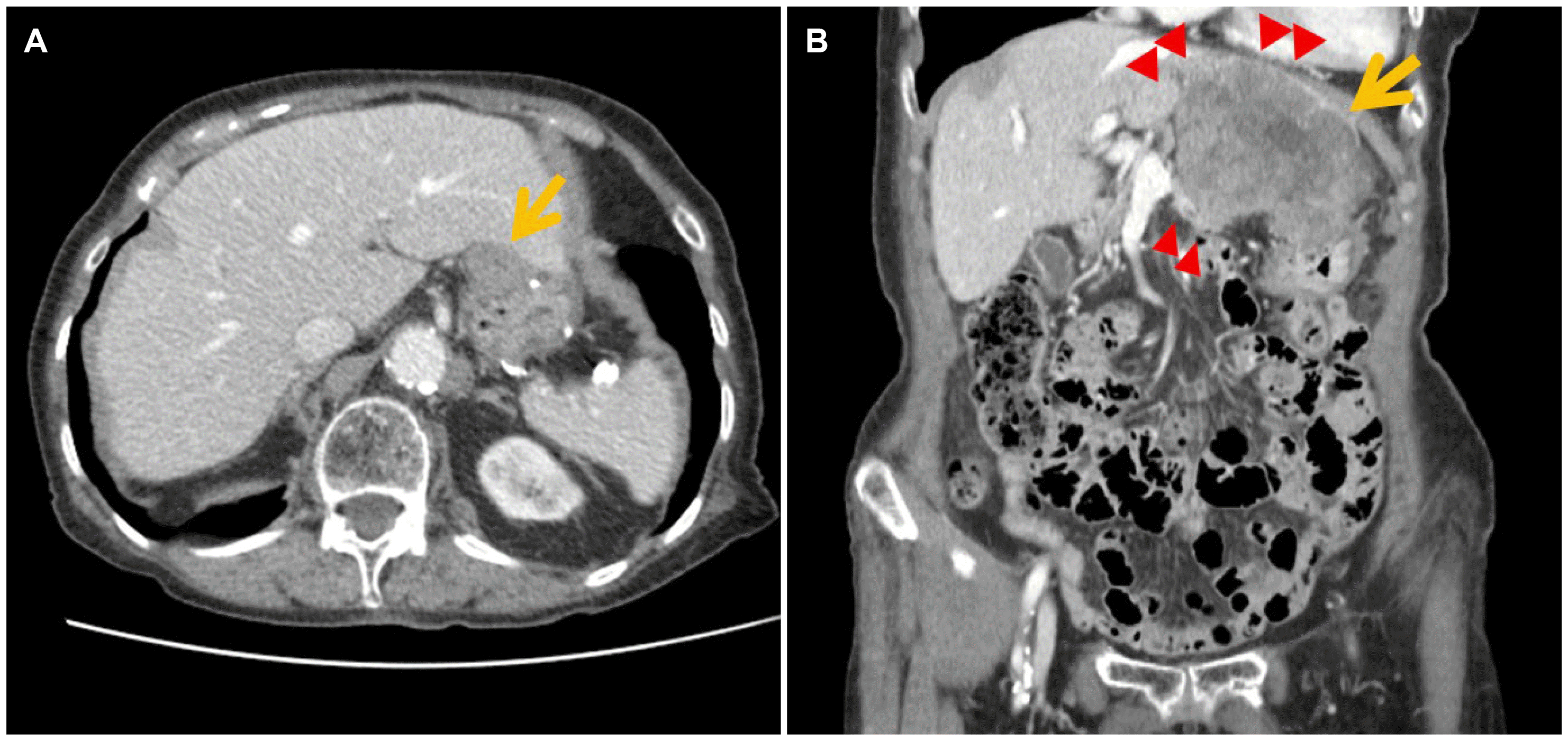 | Fig. 5Follow-up abdominal computed tomography findings after the second surgery. (A) A newly appeared 2.6-cm sized mass was found at the gastric anastomosis site (arrow), 1.5 years after the second surgery. (B) After 6 months of observation, the recurrent gastric lesion increased in size (2.6→9.4 cm, arrow) with invasion to the adjacent liver, pancreas, diaphragm, and pericardial fat (arrowheads). 
|









 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download