Abstract
Background/Aims
The incidence of stercoral perforation of the colon (SPC) is expected to rise, given the increased life expectancy and the aging population. On the other hand, the prognostic factors of mortality after surgery for SPC remain unclear. This study examined the prognostic factors of patients with SPC after surgery.
Methods
The medical records of 145 patients who underwent surgery for colonic perforation between April 2010 and May 2019 were reviewed retrospectively. In 145 patients, 22 patients who underwent SPC surgery were categorized into the following two groups according to in-hospital survival after surgery: alive (group A, n=15) and dead (group B, n=7).
Results
In all enrolled patients, the mean age was 75.7±9.0 years, with a female predominance (female patients, n=19, 86.4%). Sixteen patients (72.7%) had chronic constipation with medications, and five patients (22.7%) were bedridden. The rate of preoperative bedridden status was significantly higher in group B than group A (6.7% vs. 57.1%; p=0.021). Univariate analysis revealed immobility, a sequential organ failure assessment (SOFA) score, and lactate levels of more than 2.0 mmol/L to be factors associated with increased mortality rates in the postoperative period. Multivariate analysis revealed abnormal lactate levels to be the only factor related to mortality (hazard ratio 16.50, 95% CI 1.48-183.07, p=0.022).
Colon perforation is observed most commonly in patients with diverticulitis, malignancy, inflammatory bowel disease, or trauma. Stercoral perforation of the colon (SPC), which is defined as a perforation due to pressure necrosis from fecal mass, frequently leads to generalized peritonitis, a rare and possibly fatal critical situation.1 SPC is observed most commonly in elderly patients in immobile and moribund states with chronic constipation.2,3 The incidence of SPC is expected to rise because of the increased life expectancy and the aging population.
Although early diagnoses are rarely made because of the lack of accurate diagnostic criteria, SPC requires immediate surgery owing to the large diameter of the perforated area and its high risk of morbidity and mortality (34-57%) due to severe intraperitoneal contamination caused by feces.1,4 The risk factors of SPC have been described,3,5 but the prognostic factors of mortality after surgery for SPC were unclear. This study examined the prognostic factors of patients with SPC after surgery.
The medical charts of 145 patients, who underwent colonic perforation surgery between April 2010 and May 2019 in a single center, Dankook University Hospital, were reviewed retrospectively. The exclusion criteria were perforations caused by a malignancy (23.4%), trauma (20.0%), diverticulitis (17.2%), iatrogenic causes (9.6%), necrosis following vasculopathy (4.1%), inflammatory bowel disease (2.1%), anastomosis leak-age after colonic surgery (1.3%), and idiopathic causes (6.9%) (Table 1). After applying the exclusion criteria, 22 cases were enrolled in the study based on the radiologic features, intra-operative findings, and histopathological criteria (Table 2) (Fig. 1).6 The Institutional Review Board of Dankook University Hospital reviewed and approved this study (IRB No. 2020-07-020).
Data on the demographic parameters, perioperative findings, and pathology examination results were gathered for analysis. The demographic parameters included age, sex, BMI, comorbidities, chronic constipation, and bedridden status. The preoperative vital signs, laboratory and radiology findings, intraoperative findings, postoperative complications, and mortality were reviewed as the perioperative data. APACHE II scores were calculated as described by Knaus et al.7, and the present study referred to Ochiai et al.’s study8 for the sequential organ failure assessment (SOFA) scores. Mortality was defined as death within three months postoperatively. Moreover, the time it took to start the operation from symptom onset was also included.
A Chi-squared and Fisher’s exact test were used to analyze the categorical variables, whereas a Mann-Whitney test was applied to compare the continuous variables. Logistic regression analysis was performed to identify the risk factors for postoperative death. A p-value of more than 0.05 was considered significant. All analyses were performed using the IBM SPSS Statistics 20.0 software (SPSS Inc., Chicago, IL, USA).
Among all the patients enrolled (n=22), the mean age was 75.7±9.0 years, with a female predominance (female patients, n=19, 86.4%) and a mean BMI of 20.42±3.34 kg/m2. Patients with two or more diseases, including diabetes mellitus, hypertension, ischemic heart disease, cerebrovascular accident, and chronic kidney disease treated with dialysis, were 12 (54.5%), whereas two patients had none of these diseases. Twenty-one patients (95.4%) were identified as having the physical status of American Society of Anesthesiologists (ASA) class 3 or above in this study. Among the patients, 16 (72.7%) had chronic constipation with medications and five (22.7%) were bedridden. Fifteen patients had oral laxatives, such as magnesium hydroxide and lactulose syrup, for more than one month. Only one patient with constipation was medicated for 2 weeks. Three of five bedridden patients had chronic constipation and received rectal stimulation by suppositories or enema.
Fourteen patients (63.6%) had symptoms for more than a day, and the mean time from symptom onset to operating room transfer was 25.3±15.18 hours. A perforation site assessment revealed the sigmoid colon (n=16, 72.7%) to be the most common site, followed by the recto-sigmoid junction (n=3, 13.6%), rectum (n=2, 9.1%), and descending colon (n=1, 4.5%). The mean perforation size was 2.3±1.1, and only one patient had multiple perforations (three sites).
The patients were divided into the following two groups according to their survival after surgery: alive group A and dead group B. The alive and dead groups were statistically similar in terms of sex (female sex in group A 93.3% vs. 71.4% in group B; p=0.227), age (mean 77.0±9.0 years vs. 73.0±9.1 years; p=0.407), and BMI (mean 20.15±1.94 vs. 21.02±5.45 kg/m2; p=1.000) (Table 3). There were no differences in co-morbidity ≥2 (46.7% vs. 71.4%; p=0.381) and chronic constipation (27.3% vs. 72.7%; p=0.334) between the two groups, but the rate of preoperative bedridden status was significantly higher in group B (6.7% vs. 57.1%; p=0.021).
The time from symptom onset to surgery was similar in the two groups (mean 24.3±16.7 hours vs. 27.5±11.9 hours; p=0.616). Regarding the intraoperative findings, except for three patients who underwent a loop colostomy without resection, all surgeries (n=19, 86.4%) were performed using Hartmann’s procedure. The perforation size was similar in both groups (mean 2.3±1.3 cm vs. 2.2±0.7 cm; p=0.535).
The preoperative initial vital signs, including the mean arterial pressure, heart rates, respiratory rates, and body temperature, were similar in the two groups (Table 4). Regarding the initial laboratory findings, most of the values were similar in both groups, but the serum lactate levels were significantly higher in group B (2.96±3.17 mmol/L vs. 5.22±4.50 mmol/L; p=0.021).
An analysis of the postoperative mortality causes revealed septic shock (n=4) to be the most common cause, and the mean postoperative hospital stay of deceased patients was 8.7 days. Other reasons for death were hospital-acquired pneumonia (n=2) and acute cerebral infarction (n=1).
Univariate analysis revealed the factors associated with an increased risk of death in the postoperative period: immobility, SOFA scores, and serum lactate levels of more than 2.0 mmol/L (Table 5). Multivariate analysis revealed abnormal lactate levels to be the only factor related to mortality (hazard ratio 16.50, 95% CI 1.48-183.07, p=0.022).
Furthermore, two prognostic scoring systems were used, including APACHE II score and SOFA score. On the other hand, both scoring systems were similar in the two groups according to the multivariate analysis.
The present study identified the risk factors related to the prognosis after emergency SPC surgery. The preoperative chronic immobility and abnormal serum lactate levels may be risk factors for mortality after surgery in patients with a stercoral perforation.
SPC is a rare but dangerous condition with a mortality rate ranging from 32% to 57%.9 Berry introduced the first case of SPC to the Pathological Society of London in 1894.10 Chakravartty et al.3 observed a stercoral ulcer in 4-6% of 175 autopsies during one year. Maurer et al.6 reported seven cases and performed the first extensive review on SPC.
SPC is rare and commonly observed in elderly adults, particularly those with chronic constipation. Fecaloma from chronic constipation, which may cause SPC, expands the large bowel lumen gradually, and increases the intraluminal pressure, which results in a decrease in blood supply to the bowel wall. If not treated appropriately and the condition continues, bowel wall ulceration or perforation can occur due to ischemia.1 This is observed frequently in the sigmoid colon at a rate of 47%, followed by the rectosigmoid colon (30%) and other parts of the colon (33%).11
In the sigmoid and the rectosigmoid segments, the liquidity of feces is low, blood circulation is weak, and there is an increase in pressure due to the narrowing of the intraluminal diameter of the bowel.12 In a systematic review, 111 out of 137 (72.7%) SPC patients had chronic constipation,3 whereas in the present study, 16 out of 22 patients (72.7%) had preoperative constipation.
Except for chronic constipation, the previously identified SPC risk factors included female sex, old age, nursing facility residence, use of constipation medicine (opiates, non-steroidal anti-inflammatory drugs, anticholinergics, tricyclics), and evidence of fecal impaction.3,13-16
In this study, 86.4% of patients were female. Nam et al.4 analyzed SPC for the first time in South Korea; all eight enrolled patients were female. This trend can be explained by the following: chronic constipation is more common in the female population, and the average age of women was higher than that of men in the cohort. Women are 2-3 times more likely to have constipation than men in terms of prevalence or physical symptoms. Various theories have been proposed to explain this phenomenon. One theory states that this phenomenon is caused by a slower gut transit in women due to the changing levels of progesterone and estrogen, or damage to the pelvic floor in a women’s obstetric history.17 On the other hand, the exact cause of the higher likelihood of constipation in women is unclear. Several individuals had undergone recent surgical procedures, which may be a unifying etiology, by exacerbating constipation and contributing to nursing home stays and opiate use.
The present study showed that the patients’ mean age was 75.7 years, and the rate of emergency surgery was 100%, but the mortality rate was not higher in patients aged over 75 years. Emergency surgery is associated with high mortality rates and remains a considerable global disease burden.18 Moreover, evidence has shown that older patients have a poorer prognosis postoperatively, with the mortality rates of patients aged over 74 years being twice that of patients aged 65-74 years in one UK study.19 Physiological reserves, which are robust in younger populations, are diminished significantly in elderly patients, and cardiovascular, pulmonary, endocrine, and renal comorbidities are common. The postoperative prog-nosis may be poor, given that most SPC surgeries were performed for elderly patients in emergency settings.
Except for one patient with ASA class 2, most of the patients were identified as having ASA class 3 or 4. Univariate and multivariate analyses of emergency surgical patients and mortality revealed the ASA class to be a good predictor of death postoperatively.20 On the other hand, this is subjective and may be applied inconsistently by different anesthetists. The presence of underlying chronic conditions may deteriorate the prognosis of patients undergoing emergency surgery and may be responsible for the increased perioperative risk and mortality. The present study showed that comorbidity was not a significant prognostic factor for patients with SPC in univariate analysis (p=0.286).
Univariate analysis revealed the bedridden status before surgery to be associated with mortality after surgery (p=0.023). One recent US study developed a prediction model based on a nationwide dataset.21 A combination of risk factors associated with a more than 10% probability of survival included patients aged more than 90 years, an ASA score of 5, septic shock, and dependent functional status.
Neurological decline resulting in dementia and immunodeficiency, further complicates the diagnosis of intra-abdominal sepsis, often resulting in delays in presentation and diagnosis. Elderly or immobile patients would have recognition deficiency, dementia, and difficult communication. Therefore, some invasive procedures must be avoided. Furthermore, an enema procedure may cause colonic wall ulceration or perforation due to collisions with hard stools.22 Therefore, the role of enemas or suppositories in causing perforation and mortality must not be disregarded.
In this analysis, the times from the onset of symptoms to the transfer to admission or operating rooms were similar in the two groups. The long time interval from symptom onset to hospital admission and severe intra-abdominal contamination, resulting in increased evaporative losses and ex-tensive resuscitation, was associated with higher rates of postoperative complications.23,24 Fukuda et al.24 reported that the prognosis of patients admitted after more than 24 hours after symptom onset worsened compared to that of patients admitted within 24 hours.
Given that elderly patients with acute abdominal disease tend to have a delayed diagnosis, surgical treatments, rapid access to the hospital, adequate diagnostic measures, and quick and proper decision-making are required to prevent postoperative complications and improve the prognosis.
The systemic inflammatory response syndrome criteria assess the body temperature, heart rate, respiratory status, and leukocyte count. There were no differences in these factors. Several studies have shown that systemic inflammatory response syndrome factors cannot predict pathological changes, such as organ failure and acute circulatory failure. On the other hand, the practicality of the risk number score systems has been reported in this field. Acute physiology and chronic health evaluation II (APACHE II) scores are used as an index for patient severity assessments when admitted to the intensive care unit. Knaus et al.7 developed the APACHE II scoring system in 1985, which is comprised of 12 parameters, including blood pressure, body temperature, respiratory rate, and several laboratory data. The SOFA scores quantify the degree of organ failure and determine the mortality rate based on six aspects, including the function of respiratory, coagulation, cardiovascular, and central nervous systems as well as renal and hepatic functionalities.8 Komatsu et al.25 reported that the mortality rate was significantly higher in patients with higher APACHE-II and SOFA scores than in patients with colonic perforation. In the present study, there were no significant differences in the APACHE II and SOFA scores between the two groups.
On the other hand, the preoperative serum lactic acid levels were significantly higher in patients who did not survive than in those who did. The serum lactic acid levels reflect organ failure owing to the lack of tissue oxygenation. Therefore, they are used for a prognostic assessment of septic shock and acute respiratory distress syndrome and are useful for determining the therapeutic outcomes and changes in the pathological conditions. Many studies have reported a higher mortality associated with acidosis caused by elevated lactic acid levels than acidosis caused by other underlying causes in severe illness.26-28 In terms of colonic perforation, one study reported that the postoperative arterial blood lactic acid levels are useful for predicting the mortality rates.29
The present study had several limitations. First, this was a retrospective study performed in a single center. Therefore, selection bias may be inevitable. Obtaining accurate in-formation in emergency and operating rooms is difficult because of the retrospective nature of the study. Second, the number of enrolled cases was small (n=22), so generalizing these results to the entire population should be carefully conducted. Third, other factors associated with a postoperative prognosis, such as mental status, operative time, grade, or type of complications, were not adjusted in this study. SPC is prevalent in older patients and women with chronic constipation. Preoperative abnormal serum lactate levels are a postoperative mortality risk factor for stercoral perforation. Further research will be needed to identify the postoperative prognostic SPC factors.
REFERENCES
1. Serpell JW, Nicholls RJ. 1990; Stercoral perforation of the colon. Br J Surg. 77:1325–1329. DOI: 10.1002/bjs.1800771204. PMID: 2276009. PMCID: PMC5730425.

2. Seligman WH, Alam F, Planner A, Alexander RJ. 2016; A case of stercoral perforation detected on ct requiring proctocolectomy in a heroin-dependent patient. Case Rep Surg. 2016:2893925. DOI: 10.1155/2016/2893925. PMID: 27830103. PMCID: PMC5088268.

3. Chakravartty S, Chang A, Nunoo-Mensah J. 2013; A systematic review of stercoral perforation. Colorectal Dis. 15:930–935. DOI: 10.1111/codi.12123. PMID: 23331762.

4. Nam JK, Kim BS, Kim KS, Moon DJ. 2010; Clinical analysis of stercoral perforation of the colon. Korean J Gastroenterol. 55:46–51. DOI: 10.4166/kjg.2010.55.1.46. PMID: 20098066.

5. Gough AE, Donovan MN, Grotts J, Greaney GC. 2016; Perforated stercoral ulcer: a 10-year experience. J Am Geriatr Soc. 64:912–914. DOI: 10.1111/jgs.14057. PMID: 27100603.

6. Maurer CA, Renzulli P, Mazzucchelli L, Egger B, Seiler CA, Büchler MW. 2000; Use of accurate diagnostic criteria may increase incidence of stercoral perforation of the colon. Dis Colon Rectum. 43:991–998. DOI: 10.1007/BF02237366. PMID: 10910249.

7. Knaus WA, Draper EA, Wagner DP, Zimmerman JE. 1985; APACHE II: a severity of disease classification system. Crit Care Med. 13:818–829. DOI: 10.1097/00003246-198510000-00009. PMID: 3928249.
8. Ochiai T, Hiranuma S, Takiguchi N, et al. 2004; SOFA score predicts postoperative outcome of patients with colorectal perforation. Hepatogastroenterology. 51:1007–1010. PMID: 15239235.
9. Bunkar SK, Singh A, Singh RP. 2015; Stercoral perforation of the sigmoid colon in a schizophrenic patient. J Clin Diagn Res. 9:PD07–PD08. DOI: 10.7860/JCDR/2015/10713.5374. PMID: 25738027. PMCID: PMC4347118.

10. Berry J. 1894; Dilation and rupture of the sigmoid flexure. Br Med J. 1:301.
11. Moon JY, Hong SS, Hwang J, et al. 2019; Differentiation between stercoral perforation and colorectal cancer perforation. Rev Assoc Med Bras. 65:191–197. DOI: 10.1590/1806-9282.65.2.191. PMID: 30892443.

12. Celayir MF, Köksal HM, Uludag M. 2017; Stercoral perforation of the rectosigmoid colon due to chronic constipation: a case report. Int J Surg Case Rep. 40:39–42. DOI: 10.1016/j.ijscr.2017.09.002. PMID: 28934715. PMCID: PMC5607122.

13. Huang WS, Wang CS, Hsieh CC, Lin PY, Chin CC, Wang JY. 2006; Management of patients with stercoral perforation of the sigmoid colon: report of five cases. World J Gastroenterol. 12:500–503. DOI: 10.3748/wjg.v12.i3.500. PMID: 16489660. PMCID: PMC4066079.

14. Al Omran Y, Al Hindi S, Alarayedh S, Hawash A. 2014; Stercoral perforation in a child: a rare complication of NSAID use. BMJ Case Rep. 2014:bcr2014203652. DOI: 10.1136/bcr-2014-203652. PMID: 24658528. PMCID: PMC3962979.

15. Poitras R, Warren D, Oyogoa S. 2018; Opioid drugs and stercoral perfo-ration of the colon: case report and review of literature. Int J Surg Case Rep. 42:94–97. DOI: 10.1016/j.ijscr.2017.11.060. PMID: 29232630. PMCID: PMC5730425.

16. Davies A, Webber K. 2015; Stercoral perforation of the colon: a potentially fatal complication of opioid-induced constipation. J Pain Symptom Manage. 50:260–262. DOI: 10.1016/j.jpainsymman.2015.02.019. PMID: 25847850.
17. Leung L, Riutta T, Kotecha J, Rosser W. 2011; Chronic constipation: an evidence-based review. J Am Board Fam Med. 24:436–451. DOI: 10.3122/jabfm.2011.04.100272. PMID: 21737769.

18. Stewart B, Khanduri P, McCord C, et al. 2014; Global disease burden of conditions requiring emergency surgery. Br J Surg. 101:e9–e22. DOI: 10.1002/bjs.9329. PMID: 24272924.

19. Barlow AP, Zarifa Z, Shillito RG, Crumplin MK, Edwards E, McCarthy JM. 1989; Surgery in a geriatric population. Ann R Coll Surg Engl. 71:110.
20. Mäkelä JT, Kiviniemi H, Laitinen S. 2005; Prognostic factors of perforated sigmoid diverticulitis in the elderly. Dig Surg. 22:100–106. DOI: 10.1159/000085472. PMID: 15849471.

21. Al-Temimi MH, Griffee M, Enniss TM, et al. 2012; When is death inevitable after emergency laparotomy? Analysis of the American College of Surgeons National Surgical Quality Improvement Program database. J Am Coll Surg. 215:503–511. DOI: 10.1016/j.jamcollsurg.2012.06.004. PMID: 22789546.

22. Niv G, Grinberg T, Dickman R, Wasserberg N, Niv Y. 2013; Perforation and mortality after cleansing enema for acute constipation are not rare but are preventable. Int J Gen Med. 6:323–328. DOI: 10.2147/IJGM.S44417. PMID: 23658492. PMCID: PMC3641812.

23. McGillicuddy EA, Schuster KM, Davis KA, Longo WE. 2009; Factors predicting morbidity and mortality in emergency colorectal procedures in elderly patients. Arch Surg. 144:1157–1162. DOI: 10.1001/archsurg.2009.203. PMID: 20026835.

24. Fukuda N, Wada J, Niki M, Sugiyama Y, Mushiake H. 2012; Factors predicting mortality in emergency abdominal surgery in the elderly. World J Emerg Surg. 7:12. DOI: 10.1186/1749-7922-7-12. PMID: 22578159. PMCID: PMC3533802.

25. Komatsu S, Shimomatsuya T, Nakajima M, et al. 2005; Prognostic factors and scoring system for survival in colonic perforation. Hepatogastroenterology. 52:761–764. PMID: 15966200.
26. Gunnerson KJ, Saul M, He S, Kellum JA. 2006; Lactate versus non-lactate metabolic acidosis: a retrospective outcome evaluation of critically ill patients. Crit care. 10:R22. DOI: 10.1186/cc3987. PMID: 16507145. PMCID: PMC1550830.
27. Freire Jorge P, Wieringa N, de Felice E, Oude Lansink A, Nijsten MW, van der Horst ICC. 2017; The association of early combined lactate and glucose levels with subsequent renal and liver dysfunction and hospital mortality in critically ill patients. Crit Care. 21:218. DOI: 10.1186/s13054-017-1785-z. PMID: 28826408. PMCID: PMC5563890.

28. Jung YT, Jeon J, Park JY, Kim MJ, Lee SH, Lee JG. 2018; Addition of lactic acid levels improves the accuracy of quick sequential organ failure assessment in predicting mortality in surgical patients with complicated intra-abdominal infections: a retrospective study. World J Emerg Surg. 13:14. DOI: 10.1186/s13017-018-0173-6. PMID: 29563963. PMCID: PMC5851244.

29. Shimazaki J, Motohashi G, Nishida K, Ubukata H, Tabuchi T. 2014; Postoperative arterial blood lactate level as a mortality marker in patients with colorectal perforation. Int J Colorectal Dis. 29:51–55. DOI: 10.1007/s00384-013-1738-1. PMID: 23846515. PMCID: PMC3898377.

FIGURE AND TABLES
Fig. 1
(A) Preoperative computed tomography (CT) view of a 76-year-old female patient. This CT scan showed many fecalomas in the sigmoid colon (circle) and pneumoperitoneum around it (arrow). (B) Specimen of the same patient showed a 2 cm ovoid perforation in the sigmoid colon.
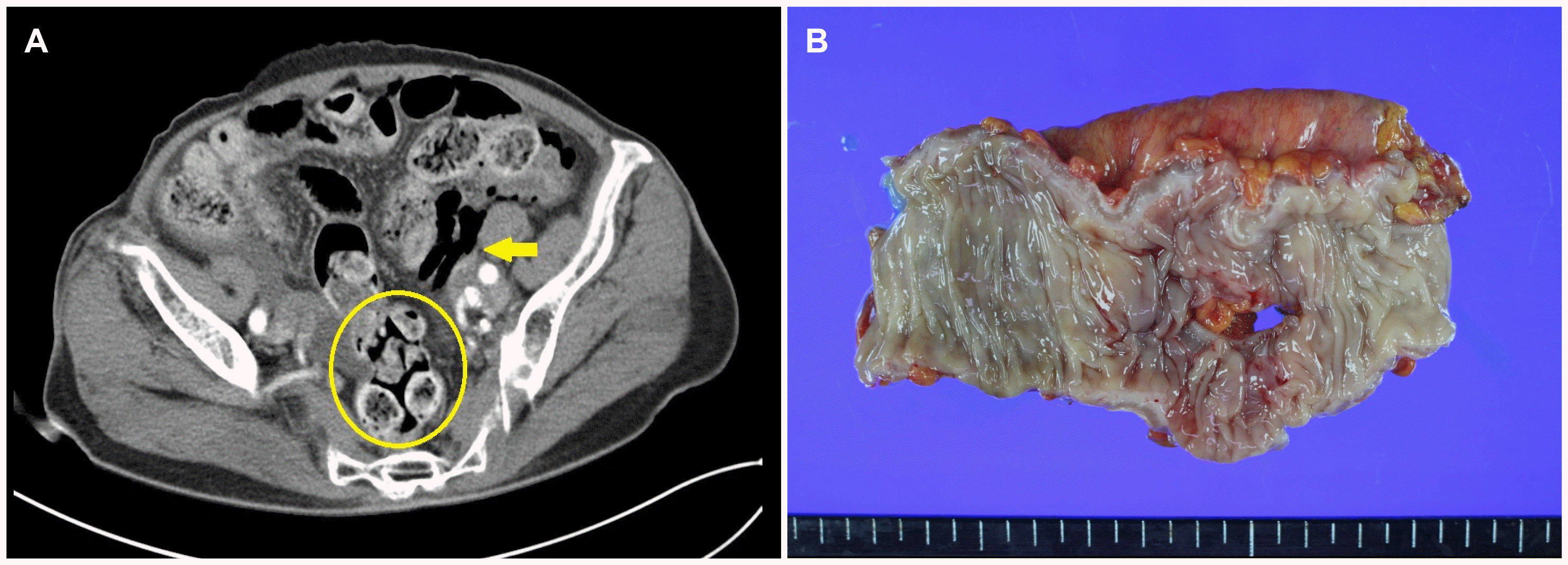
Table 1
Causes of Colon Perforation from April 2010 to May 2019 in Dankook University Hospital
Table 2
Diagnostic Criteria of Stercoral Perforation of the Colon
| 1. Round or ovoid perforation extending more than 1 cm in diameter and on the anti-mesenteric border of the bowel |
| 2. Fecalomas present within the colon, protruding through the perforation site, or lying within the peritoneal cavity |
| 3. Pressure necrosis or ulcer with chronic inflammatory reaction around the perforation site on microscopic examination |
| 4. Colonic perforations associated with another colonic pathologya were excluded |
Table 3
Characteristics of Patients who Underwent Surgery for Stercoral Perforation.
| Alive (group A) (n=15, 68.2%) | Dead (group B) (n=7, 31.8%) | p-value | |
|---|---|---|---|
| Sex | |||
| Female | 14 (93.3) | 5 (71.4) | 0.227 |
| Age (years) | 77.0±9.0 | 73.0±9.1 | 0.407 |
| BMI | 20.15±1.94 | 21.02±5.45 | 1.000 |
| Comorbidity ≥2a | 7 (46.7) | 5 (71.4) | 0.381 |
| Chronic constipation | 12 (80.0) | 4 (57.1) | 0.334 |
| Bedridden state | 1 (6.7) | 4 (57.1) | 0.021 |
| ASA class ≥3 | 14 (93.3) | 7 (100.0) | |
| Time to hospitalb | 0.193 | ||
| <24 hours | 7 (46.7) | 1 (14.3) | |
| ≥24 hours | 8 (53.3) | 6 (85.7) | |
| Time to operation roomc | 0.616 | ||
| <12 hours | 5 (33.3) | 1 (14.3) | |
| ≥12 hours | 10 (66.7) | 6 (85.7) | |
| Location of perforation | |||
| Descending colon | 1 | 0 | |
| Sigmoid colon | 11 | 5 | |
| Recto-sigmoid junction | 2 | 1 | |
| Rectum | 1 | 1 | |
| Operation type | 1 | ||
| Hartmann operation | 13 (86.7) | 6 (85.7) | |
| Loop sigmoidostomy | 2 (13.3) | 1 (14.3) | |
| Perforation size (cm) | 2.3±1.3 | 2.2±0.7 | 0.535 |
Table 4
Comparison of Preoperative Vital Signs and Laboratory Findings Between the Two Groups
Table 5
Factors Predicting Mortality in Univariate and Multivariate Analysis (n=22)




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download