This article has been
cited by other articles in ScienceCentral.
Abstract
Background/Aims
Local and systemic factors, such as diabetes, obesity, and hyperlipidemia, are considered risk factors for the recurrence of choledocholithiasis after successful endoscopic clearance. Local factors include the presence of bile sludge, common bile duct (CBD) diameter, and CBD angulation. Among them, it is unclear if acute CBD angulation is preferable to the recurrence of a CBD stone.
Methods
PubMed, EMBASE, CINAHL, the Cochrane Library databases, and google website were searched for randomized controlled trials reported in English and undertaken until August 2019. Meta‐analysis was performed on all randomized controlled trials for the recurrence of CBD stones between the patients with acute CBD angulation.
Results
Eight randomized trials (1,776 patients) were identified, and the total recurrent rate of CBD stones was 18.8% (334/1,776). A CBD angle ≤145° was significantly associated with an increased risk of recurrent CBD stone (OR=2.65, p<0.01). In two prospective studies, acute CBD angulation was not proven to be associated with a recurrence (p=0.39).
Conclusions
Approximately 20% of patients with a CBD stone showed recurrence after the complete clearance of the CBD stone, and a CBD angle ≤145° could increase the risk of recurrence. Overall, a large-scale prospective study should be necessary.
Keywords: Common bile duct, Choledocholithiasis, Recurrence
INTRODUCTION
Protective cholecystectomy is recommended to prevent biliary colic, cholecystitis, or common bile duct (CBD) stone recurrence in patients with gallbladder stones after endoscopic extraction of CBD stones.
1 On the other hand, a considerable number of CBD stone recurrences often occur, even after a cholecystectomy. The recurrence of CBD stones after ERCP, which is the treatment of choice for CBD stones, has been reported to be 4-24%.
2,3
Disease recurrence is defined when a bile duct stone reappears within 6 months of ERCP.
4 Many studies have examined the prevalence and risk factors for CBD stone recurrence after endoscopic extraction, even after a cholecystectomy. The risk factors for CBD stone recurrence are as follows: CBD diameter >15 mm, periampullary diverticulum, endoscopic sphincterotomy, sharp angulation of the CBD, history of a previous cholecystectomy, and gallbladder stone.
5-8 Several stud-ies on CBD angulation and CBD recurrence have been published since Keizman et al.
7,8 reported a link between sharp angulation (smaller than 145 on cholangiography) (
Fig. 1) and CBD stone recurrence, which would affect bile stasis.
Fig. 1
Images of a patient diagnosed with acute cholangitis and common bile duct (CBD) stones show sharp CBD angulation (119.6°, from magnetic resonance cholangiopancreatography).
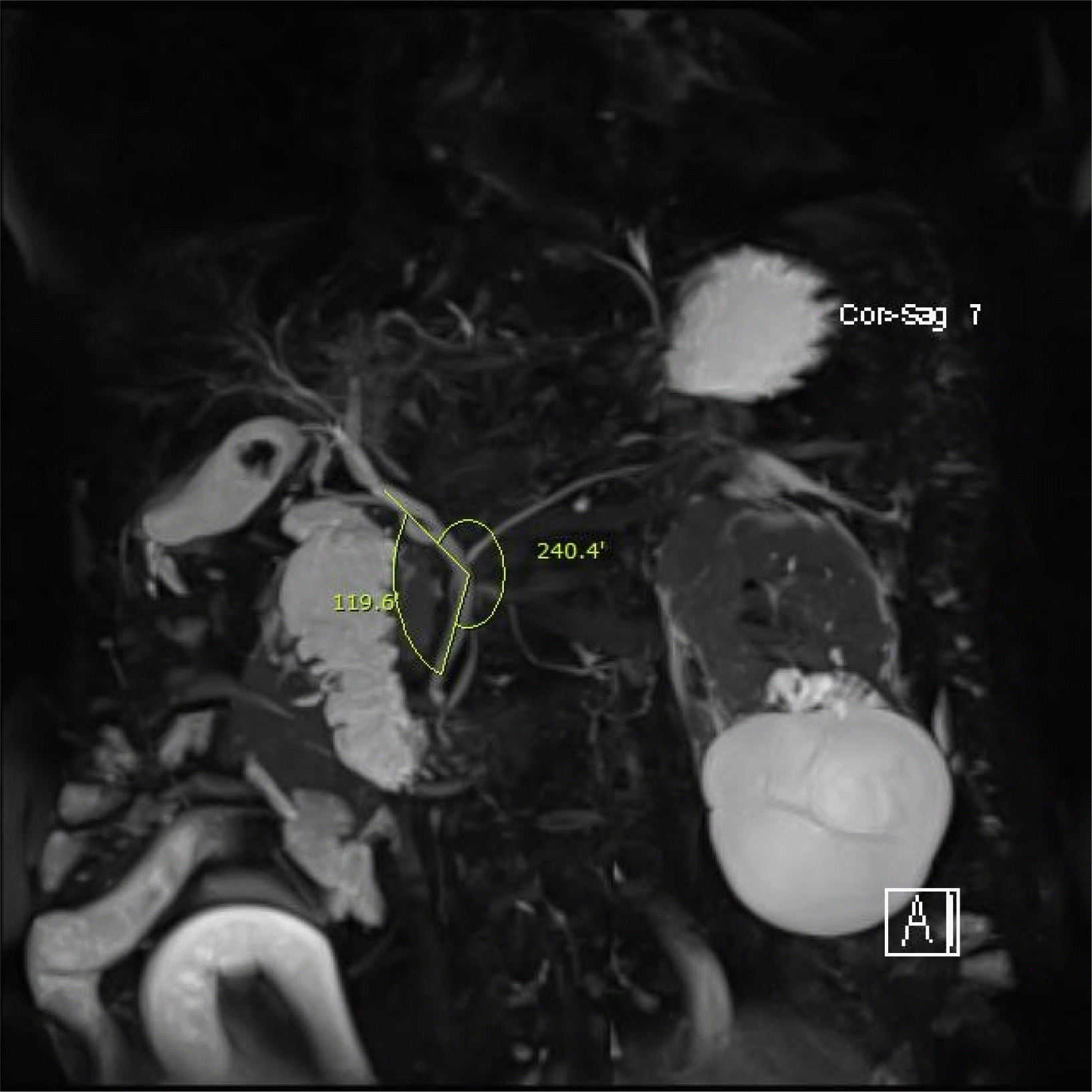

Among the risk factors of CBD stone recurrence, it is unclear if acute CBD angulation is preferable to the recurrence of a CBD stone. This systematic review and meta-analysis examined whether acute CBD angulation could be used as a predictive tool of CBD stone recurrence in patients undergoing ERCP due to gallstone diseases.
SUBJECTS AND METHODS
1. Search strategy
This systematic review and meta-analysis followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) guidelines. A systematic literature search of PubMed, EMBASE, CINAHL, and the Cochrane Library database up to May 2019 was performed. In addition to the electronic search, a manual search for additional relevant publications was also performed. The following keywords were used: (CBD angle OR CBD angulation) AND (stone recurrence). Two reviewers (YJ Kim and SH Kim) independently reviewed all potentially relevant materials to determine if they met the inclusion criteria. Ethical approval was not necessary because this article is a review.
2. Inclusion and exclusion criteria
To be included in the meta-analysis, the studies had to meet the following criteria: 1) studies conducted on human beings; 2) study population of patients with endoscopic ex-traction of CBD stones; and 3) the value of the RR, hazard ratio (HR), or OR with the corresponding 95% CIs, or the original data to calculate them were reported.
Studies were excluded if they were available only as abstracts, review studies, case reports with fewer than 10 subjects, duplicate publications, studies written in a non-English language, or if predefined outcome data required for analyses were lacking.
3. Study outcomes and data extraction
The recurrence of CBD stone was defined as the outcome, regardless of whether the measure was the primary or secondary outcome in each study. The primary outcome was the weighted summary OR of a CBD stone recurrence, according to CBD angulation with 95% CI. The secondary outcomes were to analyze and compare the odds ratios for the amount of CBD angulation, type of studies, and diagnostic methods.
Two investigators (YJ Kim and SH Kim) independently assessed the titles and abstracts of all studies retrieved and assessed their eligibility for inclusion in this meta-analysis. The reviewers used a standardized approach to conduct the literature search, data extraction, and quality assessment. The Newcastle-Ottawa scale was used to evaluate the quality of each study included. When the results were combined, discrepancies were resolved through discussion (
Supplementary Table 1).
4. Statistical analysis
The pooled odds ratio with 95% CI was evaluated for quantitative analyses. The Cochran’s Q statistics and Higgin’s I2 statistics were used to evaluate the heterogeneity of the literature. Heterogeneity was defined as follows: 1) the p-value of the Q statistics under 0.1, and 2) Higgin’s I2 of 50% or more. A random-effects model was used if heterogeneity oc-curred; otherwise, the fixed-effect model (inverse variance method) was used. A test for asymmetry of the funnel plot was performed to evaluate for publication bias. Meta-analysis of variance (ANOVA) was used to test for subgroup analysis of the type of antibiotics and the duration of the regimen. The meta-package of R language ver. 3.5.1 (R Foundation for Statistical Computing, Vienna, Austria) was performed for meta-analysis.
RESULTS
1. Identification of studies and Study characteristics
Fig. 2 shows the process of a literature search and study selection. Among the initial search strategy, 1,599 published studies were identified; eight studies are considered eligible for this meta-analysis.
5-7,9-13
Fig. 2
Flow-chart of study selection.
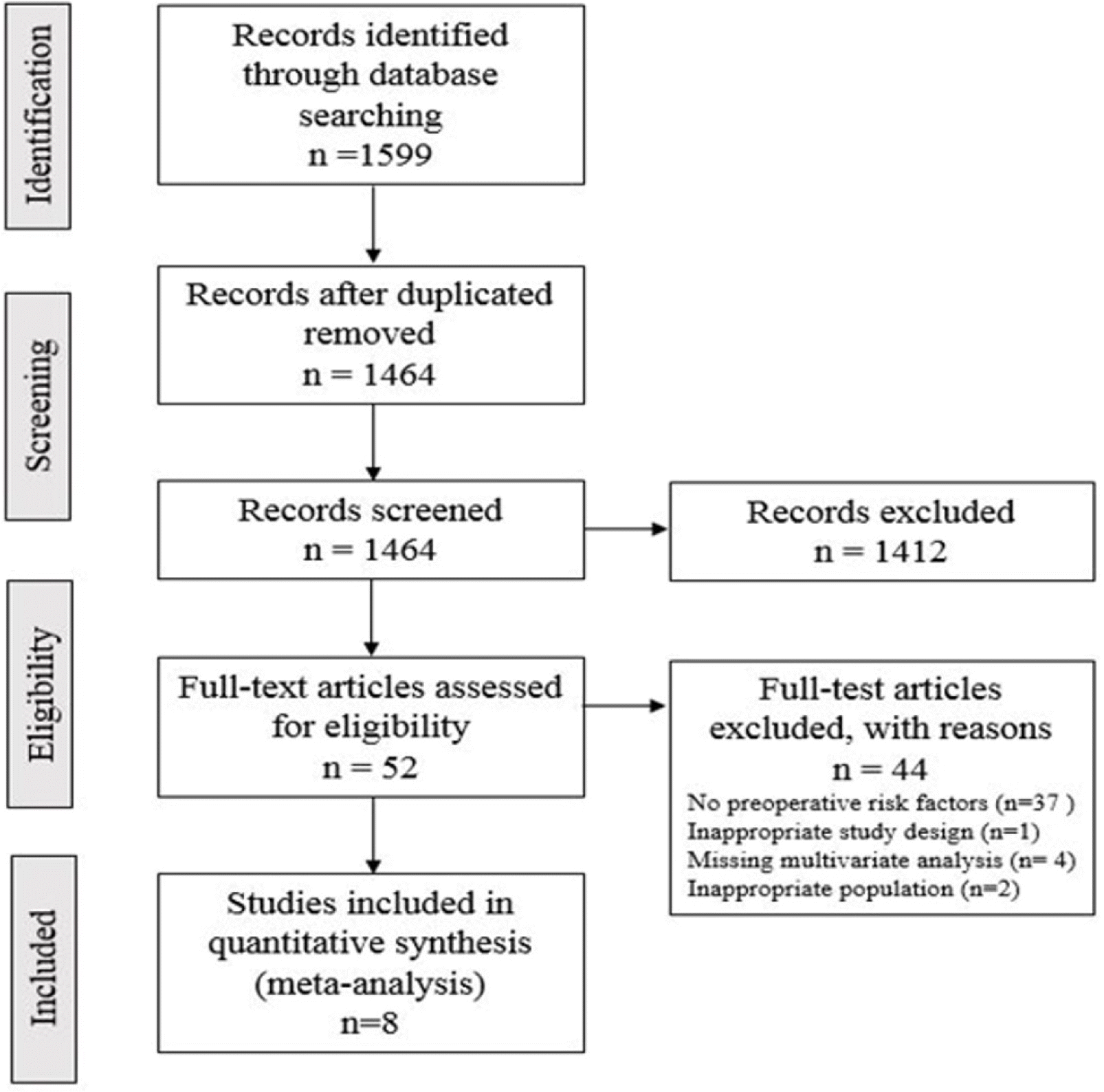

Table 1 lists the main characteristics of the included studies. The eight studies enrolled 1,776 patients with a CBD stone. Seven of these studies were conducted on Asians. The CBD angle measurements were based on 135° and 145°, respectively. The degree was divided into those measured by fluoroscopy at ERCP and those measured at MRCP.
Table 1
|
Study |
Country |
Study type |
Low CBD angle criteria |
Cholangiop ancreatography |
Non-recurrence/recurrence |
Age |
Sex (female) |
Prior cholecystectomy |
|
|
|
|
Non-recurrence |
Recurrence |
Non-recurrence |
Recurrence |
Non-recurrence |
Recurrence |
|
Zhou et al.(2019)5
|
China |
Retrospective |
Distal CBD angle ≤145° |
MRCP |
241/61 |
57.2±14.3 |
57.3±14.1 |
131 (54.4) |
33 (54.1) |
|
|
|
Yoo et al. (2018)10
|
Korea |
Retrospective |
Distal CBD angle <145° |
ERCP |
504/115 |
|
|
221 (43.85) |
61 (53.04) |
504 (100.0) |
115 (100.0) |
|
Jeon et al.(2018)12
|
Korea |
Retrospective |
Distal CBD angle ≤135° |
ERCP |
131/42 |
73.9±6.3 |
75.5±7.2 |
58 (44.27) |
17 (40.48) |
17 (13.0) |
16 (38.1) |
|
Zhang et al.(2015)9
|
China |
Prospective |
Distal CBD angle ≤135° |
MRCP |
32/32 |
56.6±14.4 |
56.6±14.4 |
23 (71.9) |
23 (71.9) |
21 (64.3) |
24 (75.0) |
|
Kim et al.(2013)6
|
Korea |
Retrospective |
Distal CBD angle <135° |
ERCP |
208/14 |
71 (37-92) |
77 (46-87) |
103 (49.52) |
7 (50.0) |
37 (17.8) |
12 (85.7) |
|
Baek et al. (2009)13
|
Korea |
Prospective |
Distal CBD angle ≤135° |
ERCP |
92/22 |
62.4±14.8 |
66.1±12.6 |
44 (47.83) |
9 (40.91) |
16 (17.4) |
7 (31.8) |
|
Keizman et al. (2006)7
|
Israel |
Retrospective |
Distal CBD angle ≤145° |
ERCP |
196/36 |
62.5±19 |
71.7±12 |
128 (65.31) |
18 (50.0) |
|
|
|
Kim et al. (2011)11
|
Korea |
Retrospective |
Distal CBD angle ≤145° |
ERCP |
38/12 |
|
|
22 (57.89) |
6 (50.00) |
12 (31.6) |
5 (41.7) |

2. CBD stone recurrence according to the CBD angulation
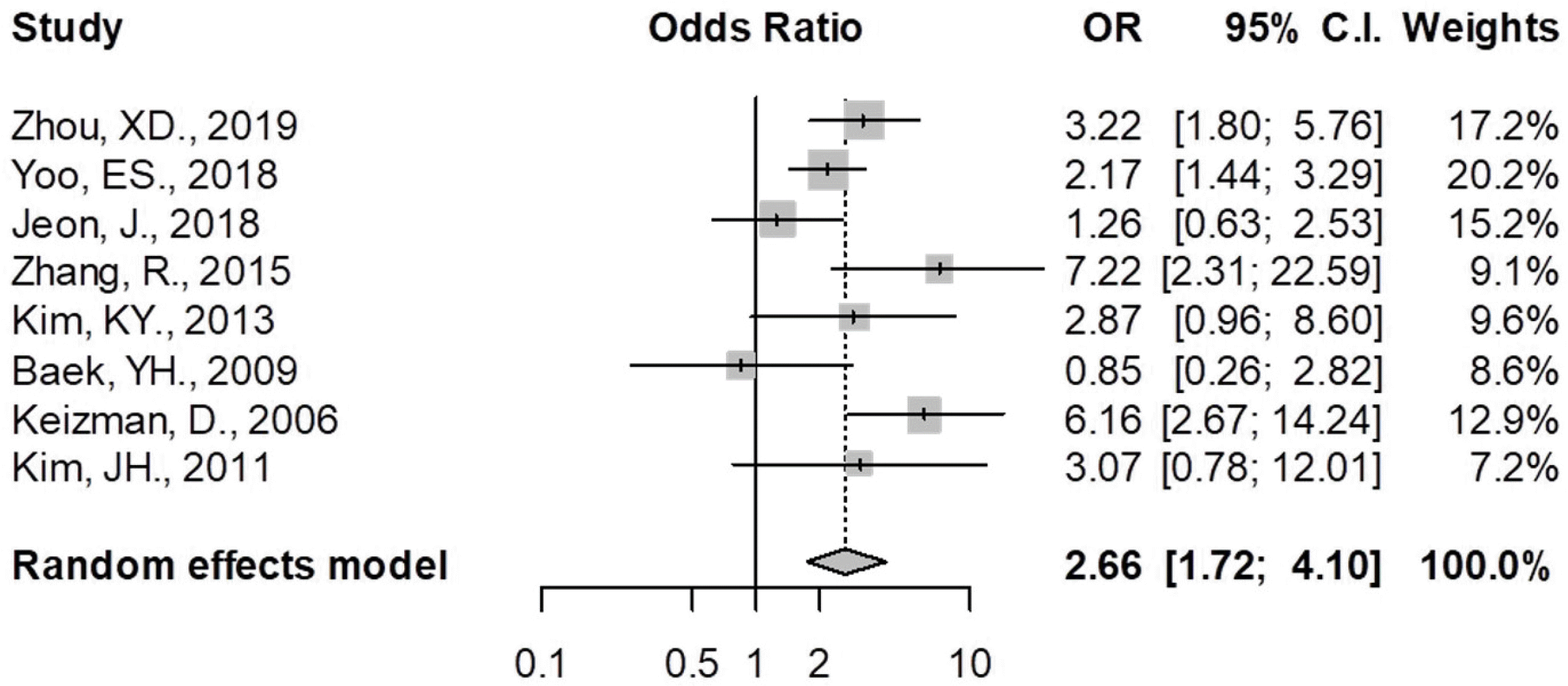
The pooled OR was 2.656 (95% CI 1.721-4.099, p<0.01), suggesting that patients with acute CBD angulation had a more than 2.6 times higher risk of disease recurrence than those without acute angulation (
Table 2, Fig 3). Subgroup analysis for the studies that selected the criteria as 135° also demonstrated a tendency for increased risk of a recurrent CBD stone, but they failed to show statistical significance (OR 2.106, 95% CI 0.883-5.020, p=0.09) (
Table 3).
Table 2
Results of Meta-analysis for the Effects of CBD Angle on Stone Recurrence
|
Study |
Non-Recurrence |
Recurrence |
OR |
95% lower CI |
95% upper CI |
Weights |
p-value |
|
|
|
Low CBD angle N |
Total N |
Proportion |
Low CBD angle N |
Total N |
Proportion |
|
Zhou et al. (2019)5
|
78 |
241 |
0.324 |
37 |
61 |
0.607 |
3.222 |
1.803 |
5.756 |
17.2 |
|
|
Yoo et al. (2018)10
|
216 |
507 |
0.426 |
71 |
115 |
0.617 |
2.174 |
1.435 |
3.293 |
20.2 |
|
|
Jeon et al. (2018)12
|
61 |
131 |
0.466 |
22 |
42 |
0.524 |
1.262 |
0.629 |
2.532 |
15.2 |
|
|
Zhang et al. (2015)9
|
6 |
32 |
0.188 |
20 |
32 |
0.625 |
7.222 |
2.309 |
22.588 |
9.1 |
|
|
Kim et al. (2013)6
|
66 |
208 |
0.317 |
8 |
14 |
0.571 |
2.869 |
0.957 |
8.601 |
9.6 |
|
|
Baek et al. (2009)13
|
19 |
92 |
0.207 |
4 |
22 |
0.182 |
0.854 |
0.258 |
2.821 |
8.6 |
|
|
Keizman et al. (2006)7
|
71 |
196 |
0.362 |
28 |
36 |
0.778 |
6.162 |
2.666 |
14.245 |
12.9 |
|
|
Kim et al. (2011)11
|
15 |
38 |
0.395 |
8 |
12 |
0.667 |
3.067 |
0.783 |
12.01 |
7.2 |
|
|
Total (random effect model) |
|
|
|
|
|
|
2.656 |
1.721 |
4.099 |
100 |
<0.0001 |

Table 3
Results of Meta-analysis for the Effects CBD Angle on Stone Recurrence (Subgroup: Low CBD Angle Criteria)
|
Study |
Non-Recurrence |
Recurrence |
OR |
95% lower CI |
95% upper CI |
Weights |
p-value |
|
|
|
Low CBD angle N |
Total N |
Proportion |
Low CBD angle N |
Total N |
Proportion |
|
135° |
|
|
|
|
|
|
|
|
|
|
|
|
Jeon et al. (2018)12
|
61 |
131 |
0.466 |
22 |
42 |
0.524 |
1.262 |
0.629 |
2.532 |
30.8 |
|
|
Zhang et al. (2015)9
|
6 |
32 |
0.188 |
20 |
32 |
0.625 |
7.222 |
2.309 |
22.588 |
23.1 |
|
|
Kim et al. (2013)6
|
66 |
208 |
0.317 |
8 |
14 |
0.571 |
2.869 |
0.957 |
8.601 |
23.8 |
|
|
Baek et al. (2009)13
|
19 |
92 |
0.207 |
4 |
22 |
0.182 |
0.854 |
0.258 |
2.821 |
22.2 |
|
|
Total (random effect model) |
|
|
|
|
|
|
2.106 |
0.883 |
5.020 |
100.0 |
0.0929 |
|
|
|
|
|
|
|
p-value of test of heterogeneity among studies=0.0313; Higgins’ I2=66.1 % (0.7%, 88.4%) |
|
145° |
|
|
|
|
|
|
|
|
|
|
|
|
Zhou et al. (2019)5
|
78 |
241 |
0.324 |
37 |
61 |
0.607 |
3.222 |
1.803 |
5.756 |
27.7 |
|
|
Yoo et al. (2018)10
|
216 |
507 |
0.426 |
71 |
115 |
0.617 |
2.174 |
1.435 |
3.293 |
54.1 |
|
|
Keizman et al. (2006)7
|
71 |
196 |
0.362 |
28 |
36 |
0.778 |
6.162 |
2.666 |
14.245 |
13.3 |
|
|
Kim et al. (2011)11
|
15 |
38 |
0.395 |
8 |
12 |
0.667 |
3.067 |
0.783 |
12.010 |
5.0 |
|
|
Total (fixed effect model) |
|
|
|
|
|
|
2.832 |
2.087 |
3.843 |
100.0 |
<0.0001 |
|
|
|
|
|
|
|
p-value of test of heterogeneity among studies=0.1669; Higgins’ I2=40.8% (0.0%, 80.0%) |

3. CBD stone recurrence according to the type of studies and measurements
Among the eight studies, only two studies were prospective. The prospective study and the retrospective study were divided and analyzed considering the potential bias of the research observer, but there was no significant difference in the prospective study (OR 2.503, 95% CI 0.309-20.283, p=0.39) (
Table 4). The analysis was divided into ERCP and MRCP cases to determine if there was a difference according to the imaging test that measured the CBD angulation. Both sub-analyses were statistically significant (
Table 5).
Table 4
Results of Meta-analysis for the Effects CBD Angle on Stone Recurrence (Subgroup: Study Type)
|
Study |
Non-Recurrence |
Recurrence |
OR |
95% lower CI |
95% upper CI |
Weights |
p-value |
|
|
|
Low CBD angle N |
Total N |
Proportion |
Low CBD angle N |
Total N |
Proportion |
|
Prospective Study |
|
|
|
|
|
|
|
|
|
|
|
|
Zhang et al. (2015)9
|
6 |
32 |
0.188 |
20 |
32 |
0.625 |
7.222 |
2.309 |
22.588 |
50.4 |
|
|
Baek et al. (2009)13
|
19 |
92 |
0.207 |
4 |
22 |
0.182 |
0.854 |
0.258 |
2.821 |
49.6 |
|
|
Total (random effect model) |
|
|
|
|
|
|
2.503 |
0.309 |
20.283 |
100.0 |
0.3902 |
|
|
|
|
|
|
|
p-value of test of heterogeneity among studies=0.0113; Higgins’ I2=84.4% (36.1%, 96.2%) |
|
Retrospective Study |
|
|
|
|
|
|
|
|
|
|
|
|
Zhou et al. (2019)5
|
78 |
241 |
0.324 |
37 |
61 |
0.607 |
3.222 |
1.803 |
5.756 |
21.8 |
|
|
Yoo et al. (2018)10
|
216 |
507 |
0.426 |
71 |
115 |
0.617 |
2.174 |
1.435 |
3.293 |
42.6 |
|
|
Jeon et al. (2018)12
|
61 |
131 |
0.466 |
22 |
42 |
0.524 |
1.262 |
0.629 |
2.532 |
15.1 |
|
|
Kim et al. (2013)6
|
66 |
208 |
0.317 |
8 |
14 |
0.571 |
2.869 |
0.957 |
8.601 |
6.1 |
|
|
Keizman et al. (2006)7
|
71 |
196 |
0.362 |
28 |
36 |
0.778 |
6.162 |
2.666 |
14.245 |
10.5 |
|
|
Kim et al. (2011)11
|
15 |
38 |
0.395 |
8 |
12 |
0.667 |
3.067 |
0.783 |
12.010 |
3.9 |
|
|
Total (fixed effect model) |
|
|
|
|
|
|
2.508 |
1.912 |
3.288 |
100.0 |
< 0.0001 |
|
|
|
|
|
|
|
p-value of test of heterogeneity among
studies=0.0918; Higgins’ I2=47.2% (0.0%, 79.1%) |

Table 5
Results of Meta-analysis for the Effects CBD Angle on Stone Recurrence (Subgroup: Cholangiopancreatography)
|
Study |
Non-Recurrence |
Recurrence |
OR |
95% lower CI |
95% upper CI |
Weights |
p-value |
|
|
|
Low CBD angle N |
Total N |
Proportion |
Low CBD angle N |
Total N |
Proportion |
|
ERCP |
|
|
|
|
|
|
|
|
|
|
|
|
Yoo et al. (2018)10
|
216 |
507 |
0.426 |
71 |
115 |
0.617 |
2.174 |
1.435 |
3.293 |
27.2 |
|
|
Jeon et al. (2018)12
|
61 |
131 |
0.466 |
22 |
42 |
0.524 |
1.262 |
0.629 |
2.532 |
20.5 |
|
|
Kim et al. (2013)6
|
66 |
208 |
0.317 |
8 |
14 |
0.571 |
2.869 |
0.957 |
8.601 |
13.1 |
|
|
Baek et al. (2009)13
|
19 |
92 |
0.207 |
4 |
22 |
0.182 |
0.854 |
0.258 |
2.821 |
11.8 |
|
|
Keizman et al. (2006)7
|
71 |
196 |
0.362 |
28 |
36 |
0.778 |
6.162 |
2.666 |
14.245 |
17.5 |
|
|
Kim et al. (2011)11
|
15 |
38 |
0.395 |
8 |
12 |
0.667 |
3.067 |
0.783 |
12.010 |
9.9 |
|
|
Total (random effect model) |
|
|
|
|
|
|
2.243 |
1.344 |
3.743 |
100.0 |
0.0020 |
|
|
|
|
|
|
|
p-value of test of heterogeneity among studies=0.0492; Higgins’ I2=55.0% (0.0%, 81.9%) |
|
MRCP |
|
|
|
|
|
|
|
|
|
|
|
|
Zhou et al. (2019)5
|
78 |
241 |
0.324 |
37 |
61 |
0.607 |
3.222 |
1.803 |
5.756 |
79.4 |
|
|
Zhang et al. (2015)9
|
6 |
32 |
0.188 |
20 |
32 |
0.625 |
7.222 |
2.309 |
22.588 |
20.6 |
|
|
Total (fixed effect model) |
|
|
|
|
|
|
3.804 |
2.268 |
6.380 |
100.0 |
< 0.0001 |
|
|
|
|
|
|
|
p-value of test of heterogeneity among
studies=0.2162; Higgins’ I2=34.6% (-, -) |

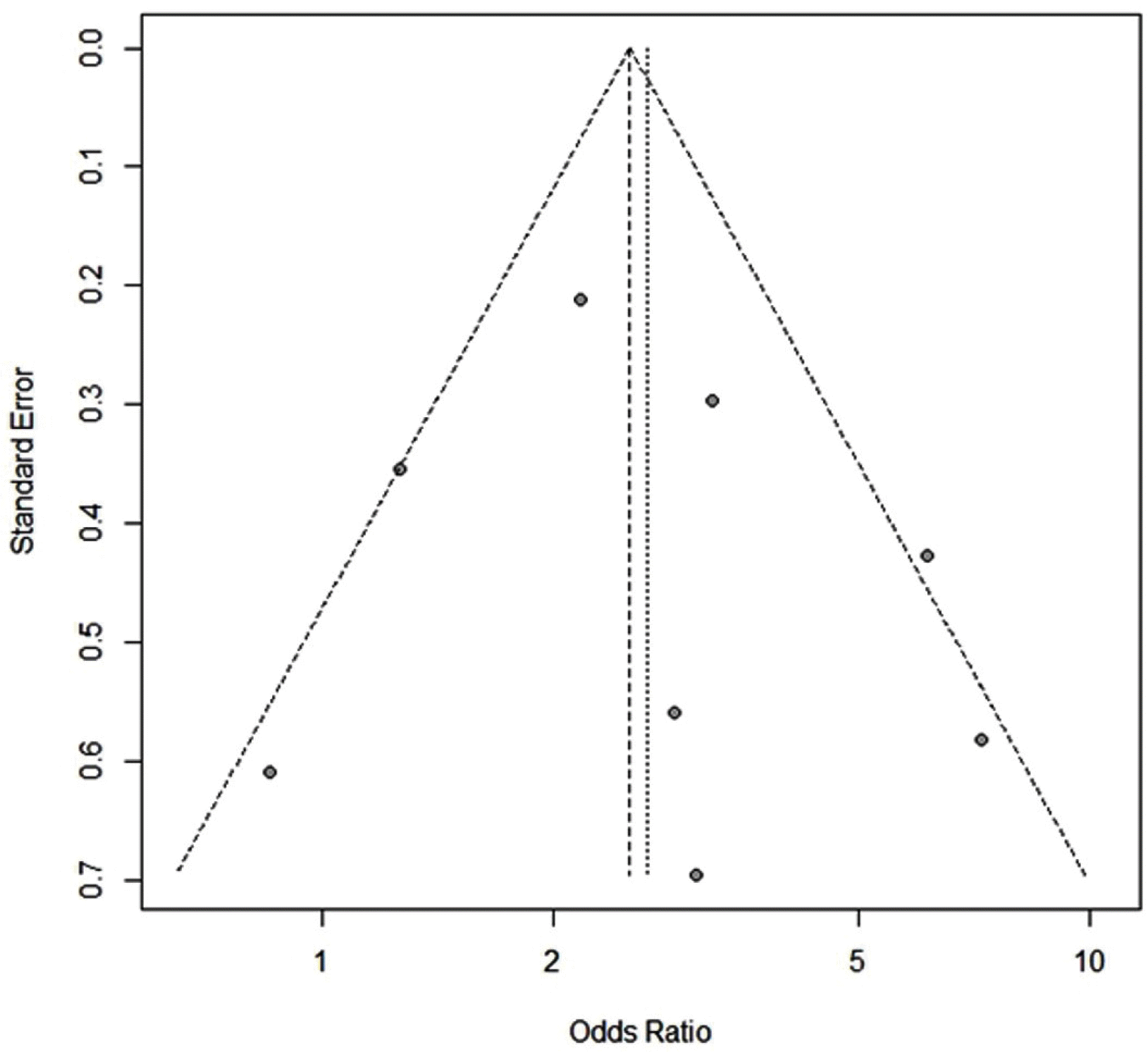
4. Publication bias
The funnel plot analysis (
Fig. 4) was symmetrical overall, demonstrating no significant publication bias (p=0.9765).
Fig. 4
Funnel plot of the study did not show publication bias.

DISCUSSION
This systematic review and meta-analysis of the clinical im-pact of CBD angulation in the recurrence of CBD stone included eight studies with 1,776 patients. The study results showed that the CBD angle ≤ 145˚ was significantly associated with an increased risk of recurrent CBD stone (OR=2.656, p< 0.01), even though it appeared to be irrelevant in cases of CBD angle ≤ 135˚ (OR=2.106, p=0.0929)
The biological mechanism for the recurrence of CBD stone on acute CBD angulation is not completely understood. Although the precise mechanism is not known, Keizman et al.
7,8 suggested that angulation along the course of the CBD promotes bile stasis, making it a risk factor for stone formation, and recurrent cholangitis after successful endoscopic therapy. Moreover, Charing et al. described the relationship between sharp RHD angulation (≤ 125˚) and acute CBD angu-lation (≤ 130˚) with recurrent cholangitis through unidentified mechanisms.
14 Therefore, further studies will be needed to clarify the mechanism that may explain the increased risk of CBD stone recurrence among acute CBD angulation.
The present systematic review and meta-analysis had some limitations. First, the outcomes of the analysis can be controversial because there were only eight articles on the association of CBD stone recurrence and acute CBD angulation. Second, except for Keizman’s study, seven observational studies were conducted in South Korea and China. Unlike western countries, the incidence of primary biliary stones in Asian countries can be relatively high compared to the number of secondary CBD stones derived from the gallbladder.
15 Therefore, it is difficult to exclude a high proportion of CBD stone recurrences in the Asian population regardless of smaller CBD angulation (≤ 145˚). Relevant studies of the western population will be needed to increase the reliability of the conclusion. Third, the cutoff values for the acute CBD angle and detailed protocols of each included study are not the same, making it difficult to generalize the result due to the increased heterogeneity of the meta-analysis. On the other hand, subgroup analysis was conducted for studies using the respective standards (135° or 145°) to reduce the unexpected effects on the results. Similar patterns were observed for the increased risk of CBD recurrence in both groups. Lastly, publication bias is likely, because all six studies were retrospective. Although no evidence of this bias was found by examining the statistical tests or funnel plots used, residual confounding factors may influence the results. The study results can be verified through further large-scale, well-organized randomized controlled trials, accompanying regional and racial diversity. Despite the above limitations, this study is meaningful in that it is the first meta-analysis of observational studies to analyze the impact of acute CBD angulation on CBD stone recurrence.
In conclusion, approximately 20% of patients with CBD stones had a recurrence after the complete clearing of the CBD stone, and a CBD angle ≤ 145˚ could increase the risk of recurrence. Therefore, MRCP surveillance of this group of patients can reduce the morbidity and mortality associated with CBD recurrence and cholangitis.




 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download