Abstract
Background/Aims
Inflammatory bowel disease (IBD) is a complex condition precipitated by genetic susceptibility and possibly a disturbed microbiome. The role of dairy foods in IBD is controversial. This study examined the association between lactose intolerance (LI) and IBD.
Methods
Data on hospital admissions of all IBD adult patients were extracted from the National Inpatient Sample database between 2004 and 2014. The comorbidities and outcomes of interest were defined by querying all the diagnostic and procedural fields for the corresponding International Classification of Diseases 9th version (ICD-9) codes. Patients with IBD were defined as the “study group,” and the patients who did not have IBD were defined as the “control group”. LI was identified in both groups using the ICD-9 codes. Multivariate logistic regression was performed to examine the association between IBD and LI.
Results
The total population was 71,342,237 patients, of which 598,129 (0.83%) had IBD. The IBD patients were younger (52 years vs. 57 years) and with fewer females (57.5% vs. 60.1%) (p<0.001 for all). After adjusting for the potential confounding factors, the IBD group had a significantly higher rate of LI (OR 2.71, 95% CI 2.55-2.88, p<0.001) compared to the non-IBD group. The findings were similar on the further stratification of IBD into Crohn’s disease compared to the control group (OR 2.70, 95% CI 2.50-2.92, p<0.001) and ulcerative colitis compared to the control group (OR 2.71, 95% CI 2.46-2.98, p<0.001).
Inflammatory bowel disease (IBD) is an umbrella term for two idiopathic chronic inflammatory diseases: ulcerative colitis (UC) and Crohn disease (CD).1 The incidence of IBD is increasing, particularly in Western countries. In North America, the incidence of UC was reported to be as high as 19.2 per 100,000 person-years, and as high as 20.2 per 100,000 per-son-years for CD.2 The pathogenesis of IBD is unclear, but may be due to a dysregulation of the immune system in response to changes in the normal gut flora.3 Additionally, multiple factors have been attributed to IBD development as well such as genetics, infections, Western diet, and smoking.4
Lactose malabsorption is present in approximately 65-70% of the population, which causes symptoms that may overlap with the symptoms of IBD, including, but not limited to, diarrhea, abdominal pain, and bloating.5 Lactose intolerance (LI) is characterized by the inability to digest lactose disaccharides.6 LI depends not only on the expression of lactase enzyme in the small intestines, but also on the dose of lactose, intestinal flora and bacterial overgrowth, gastrointestinal motility and sensitivity of the gastrointestinal tract to the generation of gas and other fermentation products of lactose malabsorption.7 Given the overlapping symptoms between IBD and LI, this study examined the association of LI with IBD using very large inpatient data.
A cross-sectional study was conducted using the National Inpatient Sample (NIS) data from 2004 to 2014. The NIS is the largest all-payer inpatient database in the USA. The database contains a sample of more than eight million inpatient stays each year, representing approximately 20% sample of discharges from all community hospitals participating in the Healthcare Cost and Utilization Project. It does not include rehabilitation and long-term acute care hospitals. Each record of the NIS data includes primary and secondary diagnoses up to 25 and primary and secondary procedures up to 15. Furthermore, it also contains the patients’ demographics, discharge status, length of stay, disease severity, and comorbidity measures.
All adult patients (≥18 years old) were included from the NIS data from 2004-2014. Using the International Classification of Diseases 9th version (ICD-9) code, all records with LI and IBD were identified using the following codes: 271.3 and 571.8, respectively. The patient demographics and comorbidities were identified using the Clinical Classification Software codes provided by the Healthcare Cost and Utilization Project, Elixhauser comorbidities, and appropriate ICD-9 codes. Table 1 lists the Elixhauser comorbidities, whereas Table 2 lists the ICD-9 codes used for LI, IBD, and other comorbidities of interest. Institutional Review Board approval was not required because NIS is a publicly available database.
The association between IBD and LI was assessed by dividing the patients into two groups: patients with IBD (study group) and patients without IBD (control group). Multivariable logistic regression analysis was performed to compare the two groups after adjusting for any potential confounding factors. Similarly, subgroup analysis was performed to assess the association between both CD and UC with LI.
The data are expressed as mean values±SD, and the frequencies were reported accordingly in percentages. Independent t-tests were used to compare the continuous variables, and a chi-square test was used for categorical variables. Multiple logistic regression was used to assess the association between LI and IBD. The regression model was adjusted for the patients’ demographics, other relevant comorbidities (obesity, smoking, and alcohol abuse), hospital characteristics, patient insurance, and socioeconomic status. p-values less than 0.05 were considered significant. SPSS version 25 software (IBM Corp, Armonk, NY, USA) was used for all statistical analyses. The data in this study were obtained from pre-existing national data and did not require approval from an Institutional Review Board.
The nationwide cohort evaluated a total of 71,342,237 patients, of which 598,129 (0.83%) had IBD and 57,909 (0.08%) had LI. The patients with IBD were younger (52 years vs. 57 years), with comparatively fewer females (57.5% vs. 60.1%) and fewer African Americans (10% vs. 14.3%) com-pared to the patients without IBD (p<0.001 for all). In addition, the patients in the IBD group had a higher prevalence of smoking (14% vs. 12%) (p<0.001) compared to the patients without IBD. On the other hand, patients in the study group were less obese (6.7% vs. 8.9%) (p<0.001) compared to the control group (details are shown in Table 3).
Multivariable logistic regression was performed after adjusting for the possible confounding factors, including age, gender, race, patient demographics, hospital characteristics, patient insurance and socioeconomic status, and other relevant comorbidities (obesity and smoking). The results showed that patients with IBD had higher odds of having LI (OR 2.71, 95% CI 2.55-2.88, p<0.001). Similarly, patients with CD and UC had a higher odds of LI: (OR 2.70, 95% CI 2.50-2.92) and (OR 2.71, 95% CI 2.46-2.98), respectively (p<0.001 for all) (forest plot is shown in Fig. 1).
Functional GI symptoms, such as abdominal pain, distention, gas, bloating, and alterations of the bowel habits, are commonly observed among patients with IBD, irritable bowel syndrome, dietary intolerance, small intestinal bacterial overgrowth, bile salt diarrhea, and other functional GI disorders.8 These functional GI symptoms may overlap with the symptoms associated with IBD-related inflammation despite achieving endoscopic and histologic mucosal healing.9 When objective evidence of active inflammation does not correlate with the persistent symptoms in IBD patients, clinicians should consider other diseases with alternate pathophysiological mechanisms, presenting with functional GI symptoms. Differentiating the symptoms of IBD-related inflammatory changes from symptoms of non-IBD diseases is important to avoid the overtreatment of IBD-related intestinal inflammation, given its as-sociated adverse effects.9
LI is a condition with an inability to digest or absorb lactose disaccharide and may occur in IBD patients because of the resulting changes in the structure and function of the GI tract related to the chronic inflammatory state of IBD.8 It is largely due to loss of the enzyme lactase phlorizin hydrolase (LPH) in the gut. The incidence of LI increases with the age with onset from 2-12 years.10 The mechanism causing the loss of lactase enzyme is unknown, even though recent studies have shown that random mutations upstream of the LCT gene could be a contributing factor.11 Owing to the inability to digest lactose, it accumulates in the gut, which is then digested by the colonic bacteria, yielding methane and other metabolites, causing symptoms related to luminal distention and stimulation of mechanoreceptors.12 Patients with IBD with concomitant LI may present with persistent GI symptoms, such as abdominal pain, bloating, fullness, and diarrhea, giving a false impression of active IBD despite the quiescent disease.13
Based on this analysis, both UC and CD patients had a significantly higher risk of having LI after adjusting for the possible confounding factors, including age, race, gender, obesity, and tobacco use. This study confirmed the findings of the largest meta-analysis by Szilagyi et al.14, which included 17 studies and 1,935 IBD patients. The risk of lactose maldigestion was reported to be higher among IBD patients than the controls. In subgroup analysis of this meta-analysis, the risk of lactose maldigestion was only significant for CD but not UC patients compared to another study, wherein both CD and UC patients were at a significantly higher risk of LI. In theishkin reported that LI prevalence in UC patients was more likely to follow the normal population, whereas CD patients had prevalence beyond the ethnic risk.15 The additional risk in CD and LI correlated with the ethnic risk, location of the disease (with the small bowel having the highest risk), and surgery.16 Mishkin et al. reported that patients with CD and lactose malabsorption, who were identified using breath-hydrogen tests, have a shorter transit time of the small bowel.15,17 In the active phases of the disease, both UC and CD patients showed improved symptoms after being given lactose-free diets.18 In UC, alterations of the small bowel mucosa may cause decreases in the disaccharidase content in the epithelium, and thus a decreased lactase enzyme level.5 Eadala et al.13, in their examination of 165 IBD patients with CC13910 genotype, reported that sensitivity to lactose occurs in a high percentage of patients in remission. They highlighted the importance of a thorough consideration of lactose malabsorption in IBD patients because of their similar symptomatology.
The hydrogen breath test remains the most common method of diagnosing lactose intolerance worldwide.19 Although a jejunal biopsy is the most accurate test for LI, its use is limited by its invasiveness.20 Genetic testing is a promising test, but its use is not sensitive in non-Caucasians and may not be suitable for assessing the secondary causes of LI.11,20 Owing to the limitations of LI diagnostic testing, a detailed history is the key to diagnosing LI, particularly in patients with IBD. Developing more reliable testing for LI is required because a LI diagnosis can be challenging in real practice.
Patients with CD may be inadvertently advised to follow dietary restrictions, such as lactose-reduced, fructose or fructan-reduced, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAP)-reduced, and gluten-free diets to help improve the functional symptoms.12 Many IBD patients are already undernourished, especially those with uncontrolled disease or in acute flares; restrictive diets can further worsen their malnutrition. Avoiding dairy products and its associated long-term effects on bone density contributes to the increasing rates of osteoporosis among IBD patients, who are already at an increased risk, particularly those on chronic steroid or immunosuppressants.14 Therefore, it is important to evaluate IBD patients for LI before subjecting them to a lactose-restricted diet, given its potential adverse effects.
To the best of the authors’ knowledge, this the largest cross-sectional study that evaluated the association between LI and IBD in a large segment of the United States population. This study had some limitations. Owing to ICD 9 limitations, NIS cannot specify if the diagnosis of LI was proven by testing. In addition, NIS relies on the accuracy of clinical data and the validity of medical diagnoses, which might differ among individuals and facilities. Furthermore, NIS cannot specify the severity of the IBD disease. Because NIS is based on inpatient data, this inclusion could lead to a larger number of sick individuals in the data, which might have affected the generalizability of the results. The exclusion of academic hospitals by the database could potentially exclude patients with more complex diseases. Regardless of these limitations, this study revealed a strong association between IBD and LI and highlighted the importance of LI screening in IBD patients. LI is a commonly observed finding among adults and is more com-mon in those who suffer from both UC and CD. Screening for LI should be considered in all IBD patients to help identify the etiology of their GI symptoms and potentially avoid unnecessary tests or changes in their maintaining regimens.
REFERENCES
1. Kappelman MD, Moore KR, Allen JK, Cook SF. 2013; Recent trends in the prevalence of Crohn's disease and ulcerative colitis in a commercially insured us population. Dig Dis Sci. 58:519–525. DOI: 10.1007/s10620-012-2371-5. PMID: 22926499. PMCID: PMC3576554.

2. M'Koma AE. 2013; Inflammatory bowel disease : an expanding global health problem. Clin Med Insights Gastroenterol. 6:33–47. DOI: 10.4137/CGast.S12731. PMID: 24833941. PMCID: PMC4020403.
3. Sairenji T, Collins KL, Evans DV. 2017; An update on inflammatory bowel disease. Prim Care. 44:673–692. DOI: 10.1016/j.pop.2017.07.010. PMID: 29132528.

4. Ananthakrishnan A, Bernstein C, Iliopoulos D, et al. 2018; Environmental triggers in IBD: a review of progress and evidence. Nat Rev Gastroenterol Hepatol. 15:39–49. DOI: 10.1038/nrgastro.2017.136. PMID: 29018271.

5. Gudmand-Hoyer E, Jarnum S. 1970; Incidence and clinical significance of lactose malabsorption in ulcerative colitis and Crohn's disease. Gut. 11:338–343. DOI: 10.1136/gut.11.4.338. PMID: 5468136. PMCID: PMC1411415.

6. Kirschner BS, DeFavaro MV, Jensen W. 1981; Lactose malabsorption in children and adolescents with inflammatory bowel disease. Gastroenterology. 81:829–832. DOI: 10.1016/S0016-5085(81)80104-7. PMID: 6895202.

7. Deng Y, Misselwitz B, Dai N, Fox M. 2015; Lactose intolerance in adults: biological mechanism and dietary management. Nutrients. 7:8020–8035. DOI: 10.3390/nu7095380. PMID: 26393648. PMCID: PMC4586575.

8. Barrett JS, Irving PM, Shepherd SJ, Muir JG, Gibson PR. 2009; Comparison of the prevalence of fructose and lactose malabsorption across chronic intestinal disorders. Aliment Pharmacol Ther. 30:165–174. DOI: 10.1111/j.1365-2036.2009.04018.x. PMID: 19392860.

9. Peyrin-Biroulet L, Reinisch W, Colombel JF, et al. 2014; Clinical disease activity, C-reactive protein normalisation and mucosal healing in Crohn's disease in the SONIC trial. Gut. 63:88–95. DOI: 10.1136/gutjnl-2013-304984. PMID: 23974954.

10. Mishkin , S . 1997; Dairy sensitivity, lactose malabsorption, and elimination diets in inflammatory bowel disease. Am J Clin Nutr. 65:564–567. DOI: 10.1093/ajcn/65.2.564. PMID: 9022546.

11. Mattar R, de Campos Mazo DF, Carrilho FJ. 2012; Lactose intolerance: diagnosis, genetic, and clinical factors. Clin Exp Gastroenterol. 5:113–121. DOI: 10.2147/CEG.S32368. PMID: 22826639. PMCID: PMC3401057.

12. Halmos EP, Christophersen CT, Bird AR, Shepherd SJ, Muir JG, Gibson PR. 2016; Consistent prebiotic effect on gut microbiota with altered FODMAP intake in patients with Crohn's disease: a randomised, controlled cross-over trial of well-defined diets. Clin Transl Gastroenterol. 7:e164. DOI: 10.1038/ctg.2016.22. PMID: 27077959. PMCID: PMC4855163.

13. Eadala P, Matthews SB, Waud JP, Green JT, Campbell AK. 2011; Association of lactose sensitivity with inflammatory bowel disease--demonstrated by analysis of genetic polymorphism, breath gases and symptoms. Aliment Pharmacol Ther. 34:735–746. DOI: 10.1111/j.1365-2036.2011.04799.x. PMID: 21815901.
14. Szilagyi A, Galiatsatos P, Xue X. 2016; Systematic review and meta-analysis of lactose digestion, its impact on intolerance and nutritional effects of dairy food restriction in inflammatory bowel diseases. Nutr J. 15:67. DOI: 10.1186/s12937-016-0183-8. PMID: 27411934. PMCID: PMC4942986.

15. Mishkin S. 1997; Dairy sensitivity, lactose malabsorption, and elimination diets in inflammatory bowel disease. Am J Clin Nutr. 65:564–567. DOI: 10.1093/ajcn/65.2.564. PMID: 9022546.

16. Mishkin B, Yalovsky M, Mishkin S. 1997; Increased prevalence of lactose malabsorption in Crohn's disease patients at low risk for lactose malabsorption based on ethnic origin. Am J Gastroenterol. 92:1148–1153. PMID: 9219788.
17. Hüppe D, Tromm A, Langhorst H, May B. 1992; Lactose intolerance in chronic inflammatory bowel diseases. Dtsch Med Wochenschr. 117:1550–1555. DOI: 10.1055/s-2008-1062476. PMID: 1396146.
18. Wright R, Truelove SC. 1965; A controlled therapeutic trial of various diets in ulcerative colitis. Br Med J. 2:138–141. DOI: 10.1136/bmj.2.5454.138. PMID: 14304053. PMCID: PMC1845668.

19. Furnari M, Bonfanti D, Parodi A, et al. 2013; A comparison between lactose breath test and quick test on duodenal biopsies for diagnos-ing lactase deficiency in patients with self-reported lactose intolerance. J Clin Gastroenterol. 47:148–152. DOI: 10.1097/MCG.0b013e31824e9132. PMID: 22495813.

20. Misselwitz B, Pohl D, Frühauf H, Fried M, Vavricka SR, Fox M. 2013; Lactose malabsorption and intolerance: pathogenesis, diagnosis and treatment. United European Gastroenterol J. 1:151–159. DOI: 10.1177/2050640613484463. PMID: 24917953. PMCID: PMC4040760.

FIGURE AND TABLES
Fig. 1
Association of IBD with LI. IBD, inflammatory bowel disease; CD, Crohn’s disease; UC, ulcerative colitis; LI, lactose intolerance; CI, confidence interval.
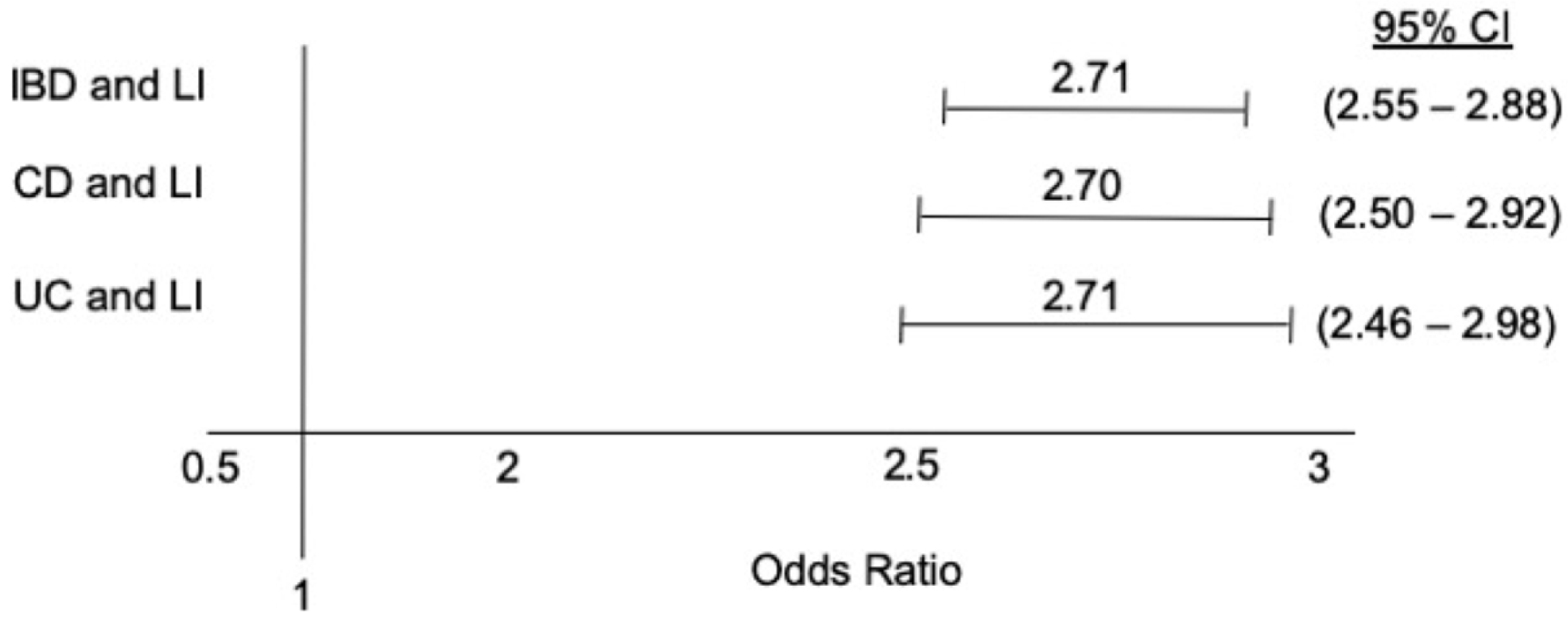
Table 2
ICD-9-CM, Clinical Modification, and CCS Codes used to Identify Comorbidities, Procedures, and Outcomes
| Variables | Source | Code(s) |
|---|---|---|
| Inflammatory bowel disease | CCS | 144 |
| Lactose intolerance | ICD-9-CM | 271.3 |
| Smoking | ICD-9-CM | 305.1 |
Table 3
Comparison of Inflammatory Bowel Disease and Non-inflammatory Bowel Disease Patients




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download