Abstract
The terminal ventricle is a dilation of the ventricular system located within the spinal cord, which is enveloped in ependymal cells that are involved in the dynamic of the cerebrospinal liquid. In the present study, four Saguinus leucopus specimens were dissected, two males and two females, whose spinal cords were extracted and histologically processed via hematoxylin and eosin stains of cuts at the conus medullaris. The S. leucopus’ terminal ventricle was observed at the conus medullaris, and had an average diameter of 241.38 μm. Thus, the presence of the terminal ventricle in the S. leucopus at the level of the conus medullaris was established.
Go to : 
The central nervous system has five ventricles [1], where the fifth is located on the most caudal (inferior) portion of the spinal cord [1, 2], and is a dilation of the central canal, which is named as terminal ventricle [2, 3]. This has been identified in a number of species, which range from rays, such as Raja clavata [4], birds as Gallus gallus [5], marsupials as Didelphis virginiana, rodents as Mus musculus and Cavia porcellus [4], ungulate as Ovis aries [6], carnivores as Canis lupus familiaris [7-9], and primates such as Macaca mulatta and Homo sapiens sapiens [10]. In H. sapiens infants, the terminal ventricle has been studied via magnetic resonance scans, which has been identified with ovoid and inconsistent appearance [11]. It is considered normal during human embryonic development [12, 13], while its presence in adults is exceptionally rare [13].
In humans, terminal ventricle is located at the conus medullaris [10-14], and is enveloped by ependymal cilia cells [3, 11], similar to Canis familiaris [9]. The terminal ventricle opens in the subarachnoid space, below the fifth sacral nerve (S5) [10]. It is typically 150 microns (0.15 mm) long by 130 microns (0.13 mm) wide, with ependymal cell cover that comes into direct contact with the pia mater, between 12.8±5.3 mm caudal to the root of the fifth sacral nerve [10]. In Macaca mulatta, the opening is located 45 mm from the S5 nerve, and is 100 μm (0.1 mm) long by 65 (0.65 mm) wide [10]. This ventricle contains cerebrospinal fluid that links to the subarachnoid space [3, 8, 10], which suggests that it may permit the drainage of cerebrospinal fluid [6]. The objective of the present investigation was to establish, for the first time, whether the Neotropical primate, Saguinus leucopus, possesses a terminal ventricle.
Go to : 
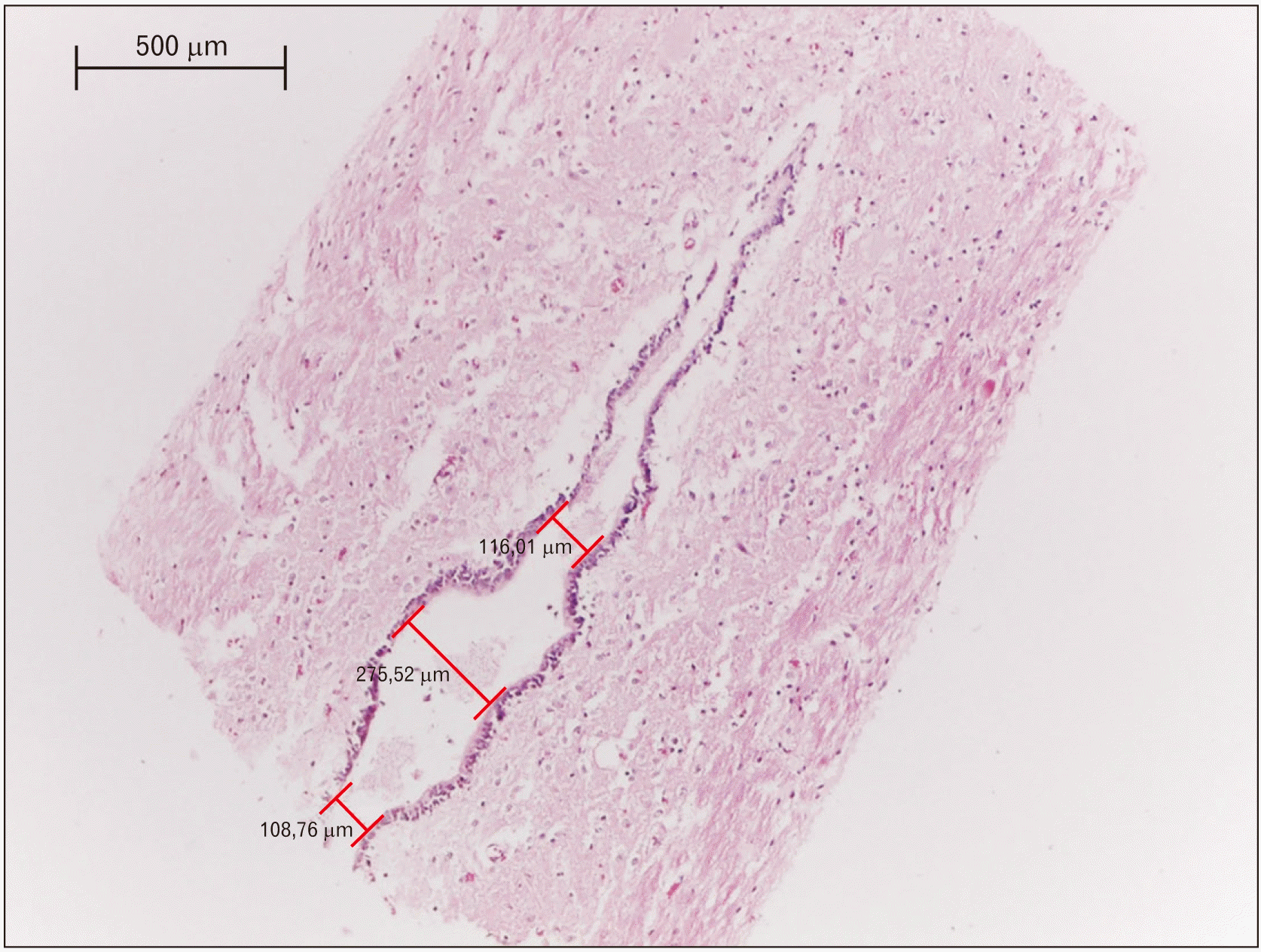
Gross dissections were performed to reach the spinal cords of four necropsied adults of S. leucopus, two males and two females, who died by natural causes in the wildlife care centers of CORPOCALDAS (Environmental authority in Caldas–Colombia). The donor entity did not report the age, body weight, and disease of the specimens. These specimens were injected with a solution of 10% formalin, 5% mineral oil, and 1% phenic acid, which was applied via subcutaneous, intramuscular, and in cavities. They were dissected to the level of the lumbar and sacral regions to remove the spinal cord (Fig. 1), which was performed separating the paravertebral muscles to extract the caudal portion of their vertebral columns. Posteriorly, the caudal extreme of the spinal cords were cut in a sagittal plane to be analyzed with a stereomicroscopy Carl Zeiss (Stemi 2000-C; Carl Zeiss Jena GmbH, Zeiss Group, Jena, Germany) associated with a camera to microphotography Carl Zeiss (AxioCam ERc 5s; Carl Zeiss Jena GmbH). Finally, the samples were dyed with a staining of hematoxylin and eosin to be analyzed and measured by optical microscope Leica DM500 associated with a camera ICC50 HD. The measurements were taken with Leica application suite version 3.4 (Fig. 2). This study was approved by the bioethics committee of the Universidad del Tolima (2.3–059).
Go to : 
The spinal cord of S. leucopus was projected caudally between the vertebrae L4 and L5, while the conus medullaris reached L5. The fifth ventricle was located there and covered by ependymal cilia cells (Fig. 2). The middle portion was the widest with an average of 241.38 μm, followed of the cranial portion with an average width of 112.54 μm and finally the caudal portion of 101.51 mm (Table 1). The opening position of terminal ventricle could not be confirmed in this study.
Go to : 
The terminal ventricle in different species is a caudal dilation of the central canal [4-6, 8-11], which is similar to that found in the present study in a Neotropical primate as S. leucopus. Therefore, the terminal ventricle could be a phylogenetic constant of the central nervous system, and the hypothesis where the terminal ventricle is postulated as a dilatation by the effect of inflammation, vascular pathology, compression, or medullar ischemia in humans [15], is a mistake. Since the terminal ventricle is an anatomical characteristic constant in other non-human animals [4, 5, 7-10]. Thus, the acknowledgment of the neuroanatomical variability is important to understand whether there are pathologic changes [16].
In infants of H. sapiens sapiens, the average length of the terminal ventricle is 22 mm, with a transverse diameter of 4.2 mm [11]. In contrast, other values oscillate between 8–10 mm of length, with transverse diameters of 4 mm are considered as a congenital dilation [17]. These values are quite different and larger than the found in S. leucopus, since these values concur with the species’ size difference, where the humans may reach an average height of 1.74 cm [18]. This would be considered large, when compared to those of S. leucopus, whose ventricle lengths, based on average body size, are between 23.74–24.88 cm, where the tail length has been excluded [19].
Go to : 
Notes
Author Contributions
Conceptualization: JEDP, MAAG, JFVG. Data acquisition: JEDP, MAAG. Data analysis or interpretation: JEDP, MAAG, JFVG. Drafting of the manuscript: JEDP, MAAG, JFVG. Critical revision of the manuscript: JEDP, MAAG, JFVG. Approval of the final version of the manuscript: all authors.
Go to : 
References
1. Duque-Parra JE, Barco-Ríos J, García-Aguirre JF. 2017; A historical approach to the ventricular system of the brain. Rev Fac Med. 65:473–7. DOI: 10.15446/revfacmed.v65n3.57884.

2. Woodley-Cook J, Konieczny M, Spears J. 2016; The slowly enlarging ventriculus terminalis. Pol J Radiol. 81:529–31. DOI: 10.12659/PJR.895669. PMID: 27867442. PMCID: PMC5102252.

3. Liccardo G, Ruggeri F, De Cerchio L, Floris R, Lunardi P. 2005; Fifth ventricle: an unusual cystic lesion of the conus medullaris. Spinal Cord. 43:381–4. DOI: 10.1038/sj.sc.3101712. PMID: 15655569.

4. Vigh B, Vigh-Teichmann I, Manzano e Silva MJ, van den Pol AN. 1983; Cerebrospinal fluid-contacting neurons of the central canal and terminal ventricle in various vertebrates. Cell Tissue Res. 231:615–21. DOI: 10.1007/BF00218119. PMID: 6871973.

5. Uehara M, Ueshima T. 1985; Light and electron microscopy of the chicken coccygeal cord. Nihon Juigaku Zasshi. 47:963–70. DOI: 10.1292/jvms1939.47.963. PMID: 4094279.

6. Storer KP, Toh J, Stoodley MA, Jones NR. 1998; The central canal of the human spinal cord: a computerised 3-D study. J Anat. 192(Pt 4):565–72. DOI: 10.1046/j.1469-7580.1998.19240565.x. PMID: 9723983. PMCID: PMC1467810.

7. Fitzgerald P. 1967; The fifth ventricle. Ir J Med Sci. 6:133–6. DOI: 10.1007/BF02954269. PMID: 6042012.

8. Marín-García P, González-Soriano J, Martinez-Sainz P, Contreras-Rodríguez J, Del Corral-Gros C, Rodríguez-Veiga E. 1995; Spinal cord central canal of the German shepherd dog: morphological, histological, and ultrastructural considerations. J Morphol. 224:205–12. DOI: 10.1002/jmor.1052240209. PMID: 7745605.

9. Fletcher TF. Evans H, de Lahunta A, editors. 2013. Spinal cord and meninges. Miller's anatomy of the dog. 4th ed. Elsevier;Louis: p. 589–610.
10. Sakata M, Yashika K, Hashimoto PH. 1993; Caudal aperture of the central canal at the filum terminale in primates. Kaibogaku Zasshi. 68:213–9. PMID: 8337935.
11. Coleman LT, Zimmerman RA, Rorke LB. 1995; Ventriculus terminalis of the conus medullaris: MR findings in children. AJNR Am J Neuroradiol. 16:1421–6. PMID: 7484626.
12. Lotfinia I, Mahdkhah A. 2018; The cystic dilation of ventriculus terminalis with neurological symptoms: three case reports and a literature review. J Spinal Cord Med. 41:741–7. DOI: 10.1080/10790268.2018.1474680. PMID: 29791269. PMCID: PMC6217512.

13. Suh SH, Chung TS, Lee SK, Cho YE, Kim KS. 2012; Ventriculus terminalis in adults: unusual magnetic resonance imaging features and review of the literature. Korean J Radiol. 13:557–63. DOI: 10.3348/kjr.2012.13.5.557. PMID: 22977322. PMCID: PMC3435852.

14. Rossi A, Biancheri R, Cama A, Piatelli G, Ravegnani M, Tortori-Donati P. 2004; Imaging in spine and spinal cord malformations. Eur J Radiol. 50:177–200. DOI: 10.1016/j.ejrad.2003.10.015. PMID: 15081131.

15. Nassar SI, Correll JW, Housepian EM. 1968; Intramedullary cystic lesions of the conus medullaris. J Neurol Neurosurg Psychiatry. 31:106–9. DOI: 10.1136/jnnp.31.2.106. PMID: 5684018. PMCID: PMC496312.

16. Nayak SB, Shetty SD. 2019; Partial duplication of tentorium cerebelli and complete duplication of falx cerebelli. Anat Cell Biol. 52:337–9. DOI: 10.5115/acb.19.017. PMID: 31598364. PMCID: PMC6773897.

17. Sigal R, Denys A, Halimi P, Shapeero L, Doyon D, Boudghène F. 1991; Ventriculus terminalis of the conus medullaris: MR imaging in four patients with congenital dilatation. AJNR Am J Neuroradiol. 12:733–7. PMID: 1882755.
18. Bogin B, Varela-Silva MI. 2010; Leg length, body proportion, and health: a review with a note on beauty. Int J Environ Res Public Health. 7:1047–75. DOI: 10.3390/ijerph7031047. PMID: 20617018. PMCID: PMC2872302.

19. Castañeda FE, Buritica EF, Barbosa IX. 2010; White-footed tamarin Saguinus leucopus GUNTHER 1876: some biological aspects and issues of veterinary interest about the species. Rev Colombiana Cienc Anim. 3:81–9.
Go to : 




 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download