Introduction
Materials and Methods
Cells and cell culture conditions
Reagents and antibodies
Reverse-transcription polymerase chain reaction
Western blotting
Evaluation of cell viability
Detection of apoptosis by Annexin V/propidium iodide
Measurement of cytochrome c and Bax translocation
Detection of sub-G1 level
Statistical analysis
Results
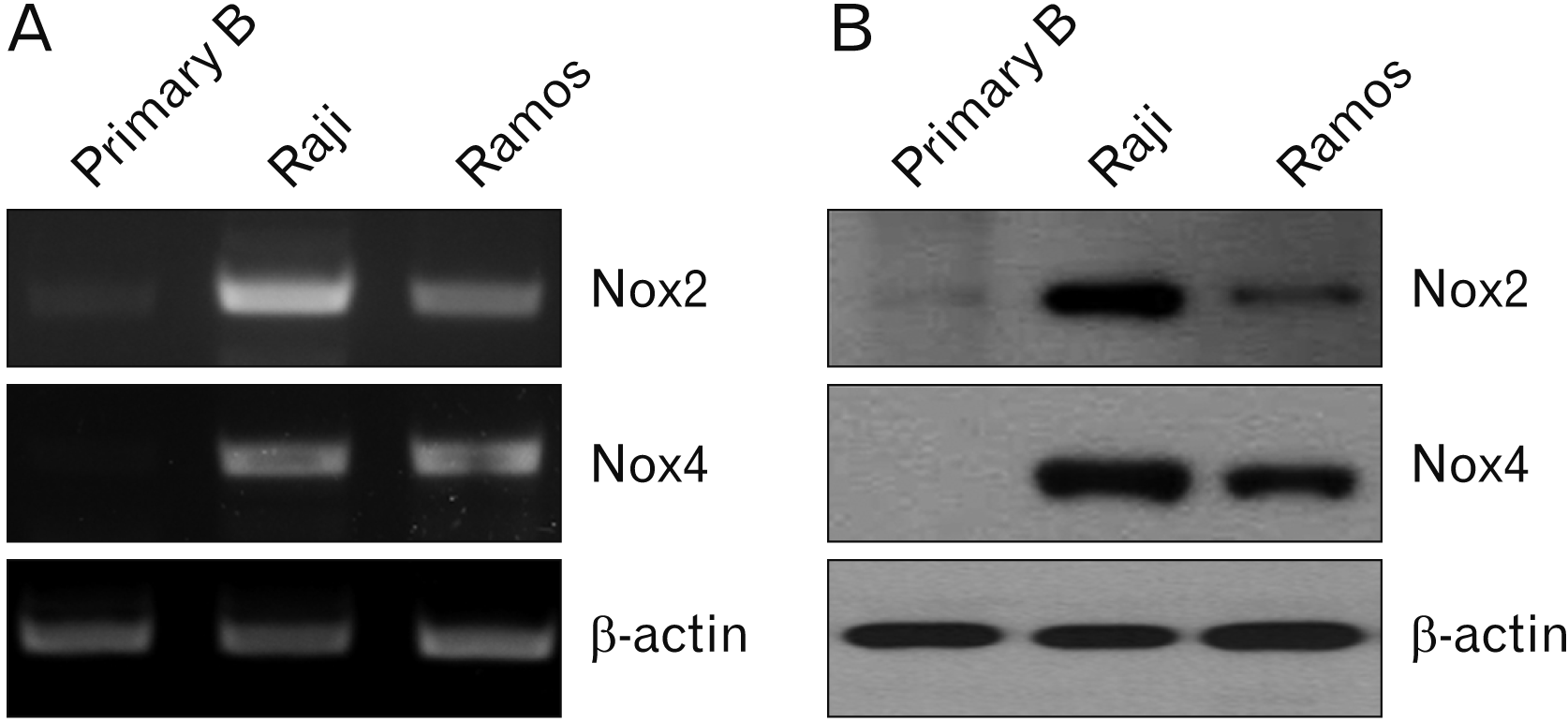
Nox2 and Nox4 were highly expressed in Raji cells compared with primary B and Ramos cells
 | Fig. 1Cell viability changes at 48 hours after Nox2 inhibitor, GSK2795039, treatment. Cells were seeded and treated with GSK2795039 at the indicated doses for 48 hours. Cell viability was analyzed using a WST-1 assay or trypan blue exclusion method. (A) Primary B cells, (B) Raji cells, (C) Ramos cells. Nox2, nicotinamide adenine dinucleotide phosphate oxidase 2; Primary B, primary B cells. The data shown are representative of three independent experiments (*P<0.05 vs. control, **P<0.01 vs. control). |
Nox2 inhibitor treatment induced cell death in Raji cells
 | Fig. 2PCR and western blot analyses of NADPH oxidase expression in primary B, Raji, and Ramos cells. (A) NADPH oxidase mRNA expression was verified by RT-PCR. β-actin was used as a loading control. Cells were harvested, and PCR amplification was performed as described as in the Materials and Methods. (B) Cells were harvested and cell lysates were extracted. The cell lysates were subjected to western blot using anti-human Nox2, -4, and β-actin antibodies. β-actin was used as a loading control. Results are representative of three independent experiments. NADPH, nicotinamide adenine dinucleotide phosphate; Nox2, NADPH oxidase 2; Nox4, NADPH oxidase 4; PCR, polymerase chain reaction; Primary B, primary B cells; RT, reverse-transcription. |
Nox2 inhibitor induced apoptosis in Raji cells in time- and dose-dependent manners
 | Fig. 3Apoptosis analysis of Raji cells after Nox2 inhibitor, GSK2795039 treatment. Raji cells were treated with GSK2795039 for 48 hours. Cells were harvested, washed, and stained with FITC-conjugated Annexin V and PI as described in the Materials and Methods. Stained cells were analyzed by flow cytometry. The numbers in box represents the cell proportion in each quadrant. Data are representative of the three independent experiments. DMSO, control for GSK2795039; Negative, negative control; Nox2, nicotinamide adenine dinucleotide phosphate oxidase 2; PI, propidium iodide. |
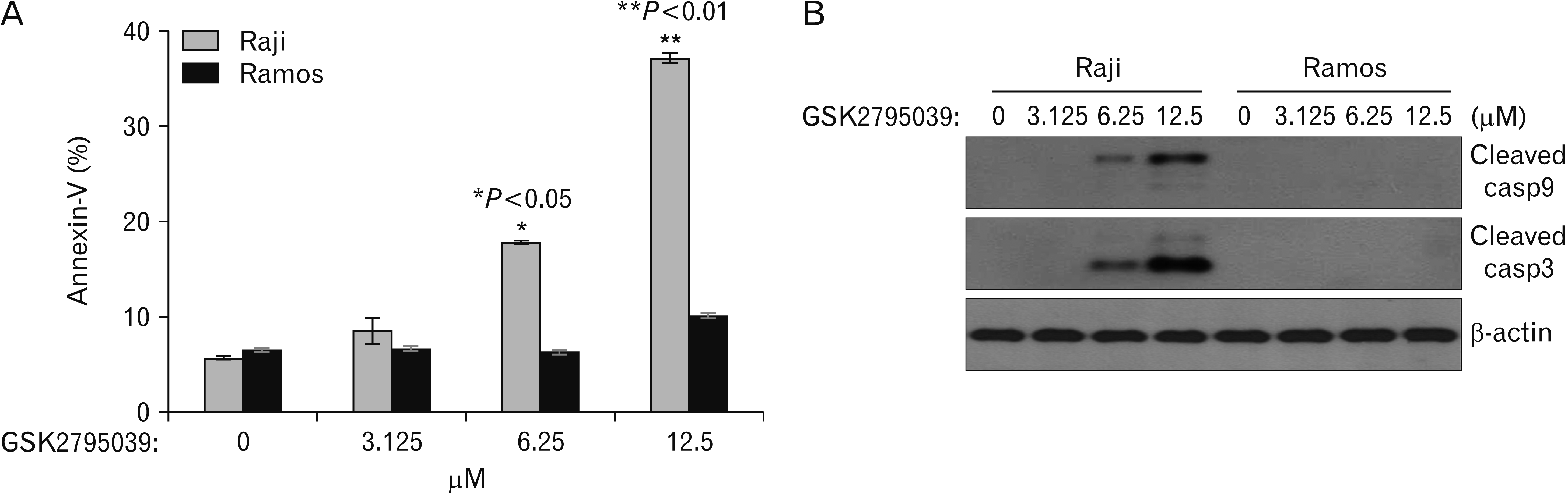 | Fig. 4GSK2795039, the Nox2 inhibitor, mediated caspase-dependent apoptotic cell death in Raji cells in a dose-dependent manner. (A) Raji and Ramos cells were treated with GSK2795039 in various concentrations for 48 hours and then stained with Annexin V-FITC. Apoptotic cell populations were determined with flow cytometry. * and ** indicate P<0.05 and P<0.01, respectively, compared with the Ramos cell apoptotic population. (B) Western blot analysis of caspase activation in cells after GSK2795039 treatment. β-actin was used as a loading control. Cell graphs represent the mean±standard deviation of three independent experiments in triplicate. Red box represents meaningful changes of molecule expressions. Nox2, nicotinamide adenine dinucleotide phosphate oxidase 2. |
Caspases are involved in Nox2 inhibitor-induced apoptosis
Mcl-1, Bim, and Noxa are involved in Nox2 inhibitor induced-apoptosis of Raji cells
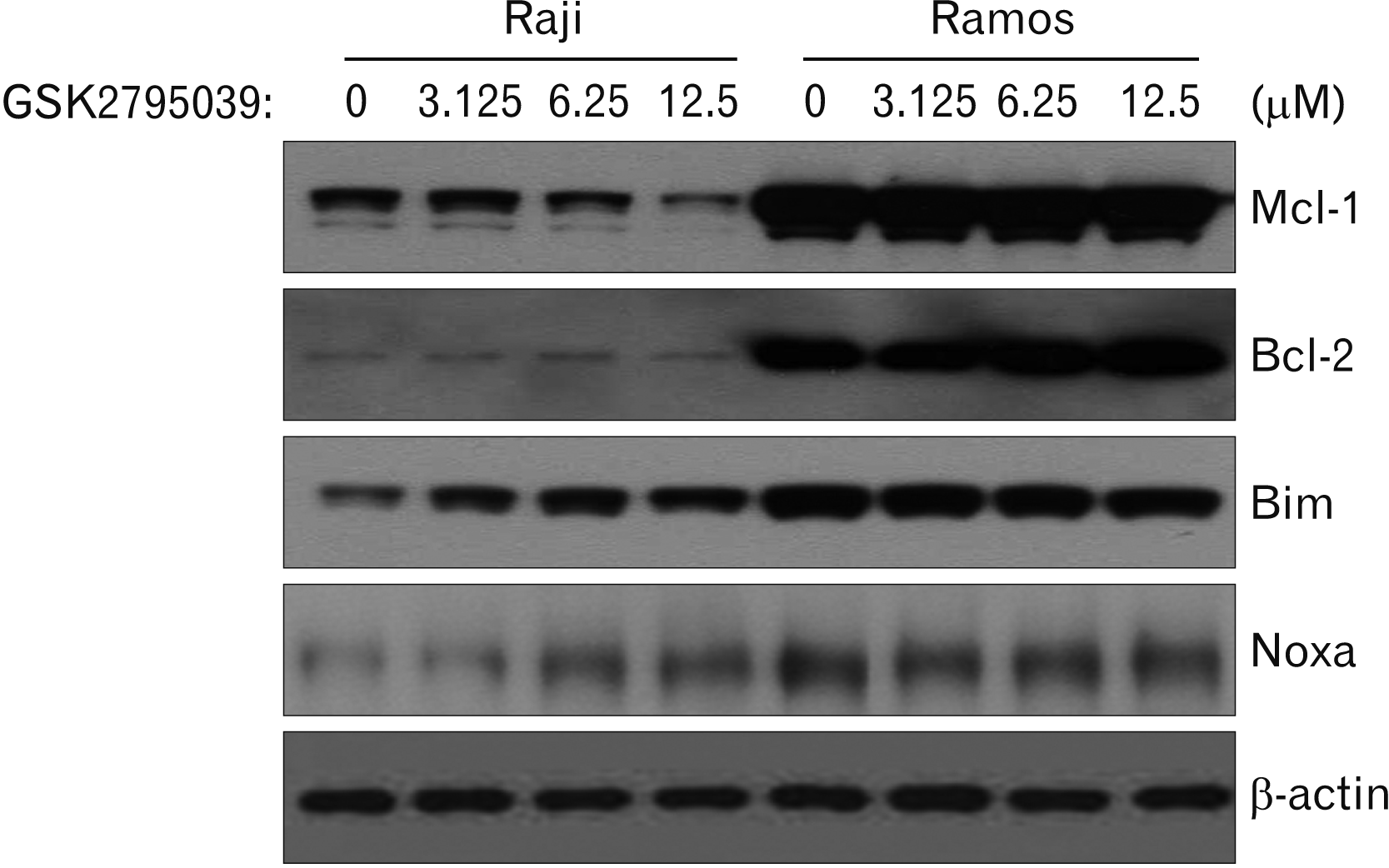 | Fig. 5Effects of the Nox inhibitor, GSK2795039, on Bcl-2 family proteins expressions in Raji and Ramos cells. After GSK2795039 treatment, cells were harvested and cell lysates were extracted. The cell lysates were subjected to western blot using anti-human Mcl-1, Bcl-2, Bim, Noxa, and β-actin antibodies. β-actin was used as a loading control. Results are representative of three independent experiments. Red box represents meaningful changes of molecule expressions. |
Translocation of Bax and release of cytochrome c in Raji cells after GSK2795039 treatment
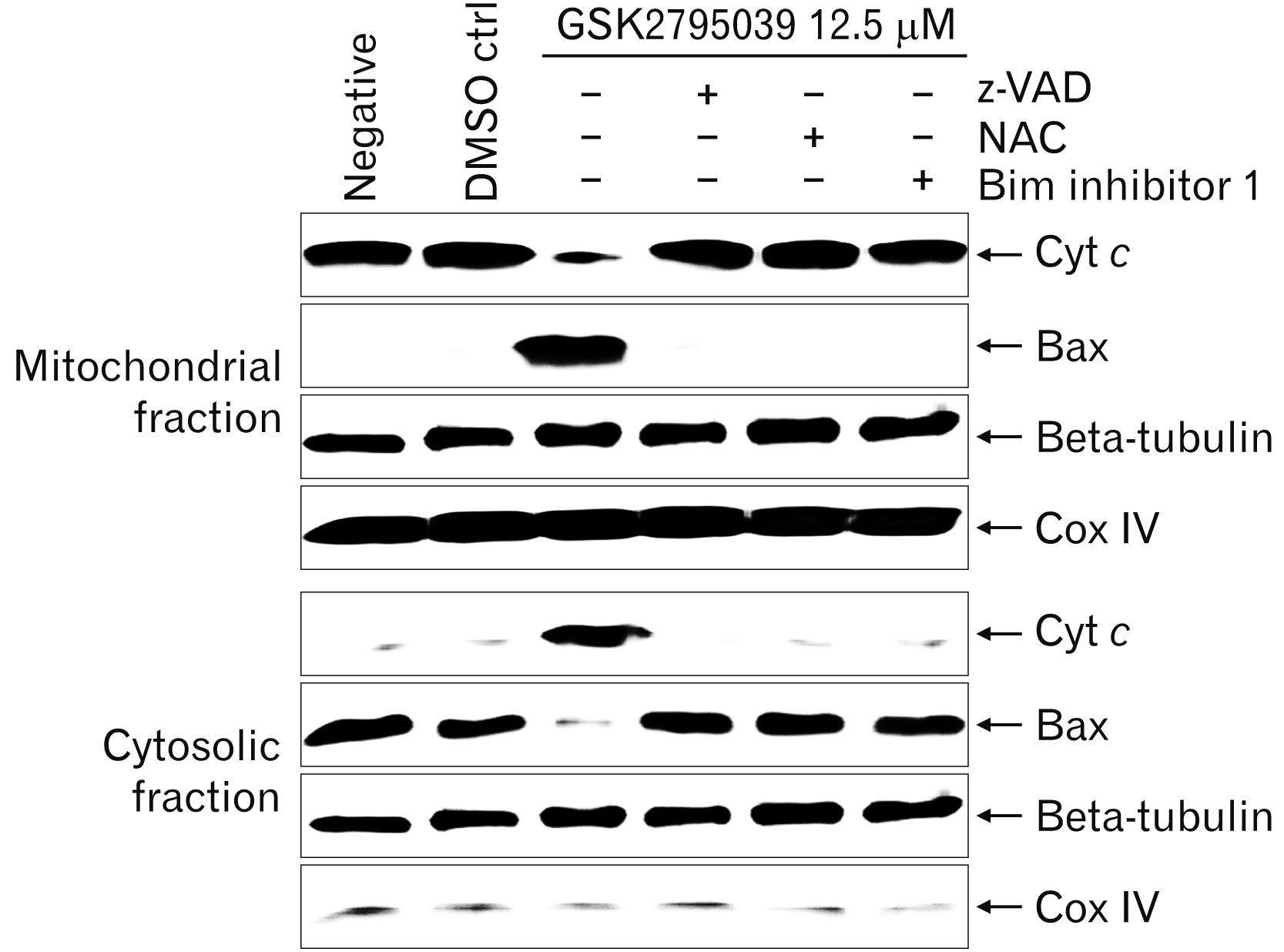 | Fig. 6Effects of GSK2795039 on the translocation of Bax and release of Cyt c in Raji cells. After GSK2795039 treatment, cells were harvested and cell lysates were extracted. The cell lysates were separated into cytosolic and mitochondrial fractions, which were then analyzed by western blotting using anti-human Cyt c, bax, beta-tubulin, and Cox IV antibodies. Anti-Cox IV and anti-beta tubulin antibodies were used for fraction loading controls. Results are representative of three independent experiments. Cyt c, cytochrome c; DMSO ctr, solvent control; GSK2795039, Nox2 inhibitor; NAC, N-acetyl-L-cysteine, reactive oxygen species scavenger; Negative, negative control without treatment; z-VAD, N-benzyloxycarbonyl-Val-Ala-Asp, a pan-caspase inhibitor. |
Inhibition assay using z-VAD, NAC, and BI-1 in Raji cells after GSK2795039 treatment
 | Fig. 7Inhibitory effects of z-VAD, NAC, and Bim inhibitor 1 on the changes of sub-G1 populations induced by GSK2795039 in Raji cells. To assess apoptotic cells, cells were stained with PI as described in the Materials and Methods. Cells were then analyzed by flow cytometry. The percentage represents the fraction of sub-G1 cells (M1) relative to the total number of cells. Results are representative of three independent experiments. DMSO ctr, solvent control; GSK2795039, Nox2 inhibitor; NAC, N-acetyl-L-cysteine, reactive oxygen species scavenger; Negative, negative control without treatment; z-VAD, N-benzyloxycarbonyl-Val-Ala-Asp, pan-caspase inhibitor. |
Discussion
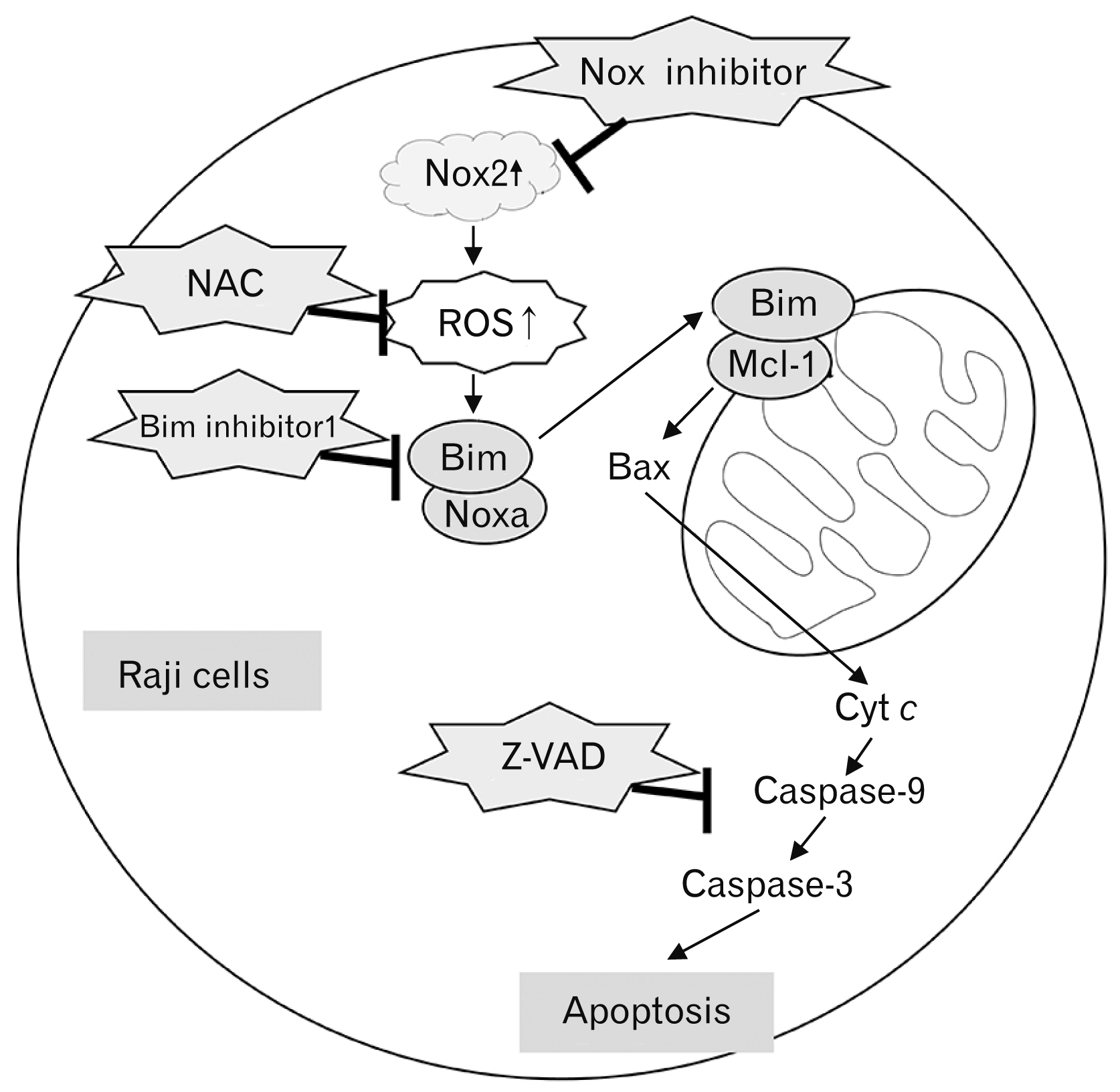 | Fig. 8Schematic diagram of the intracellular signaling mechanism during Nox2 inhibitor-induced apoptosis in Raji cells. The increase of Nox2 expression effectively causes ROS generation as an initial response in EBV-positive B lymphoma Raji cells. Both the Nox inhibitor and ROS scavenger NAC effectively inhibit the cellular response following ROS generation. Aberrant ROS-mediated Bim and Noxa activation and Mcl-1 down-regulation induce the translocation of Bax to the mitochondria membrane, resulting in mitochondrial membrane potential disruption. The Bax pores cause the release of Cyt c into the cytoplasm, resulting in caspase cascade activation and apoptosis. Cyt c, cytochrome c; EBV, Epstein-Barr virus; NAC, N-acetyl-L-cysteine; Nox2, nicotinamide adenine dinucleotide phosphate oxidase 2; ROS, reactive oxygen species. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download