Introduction
Materials and Methods
Chemicals and drugs
Animals
Experimental design
Histopathological examination
Ultrastructure examination
Immunohistochemical examination
Morphometric and statistical analysis
Results
Histopathological findings
hematoxylin and eosin staining
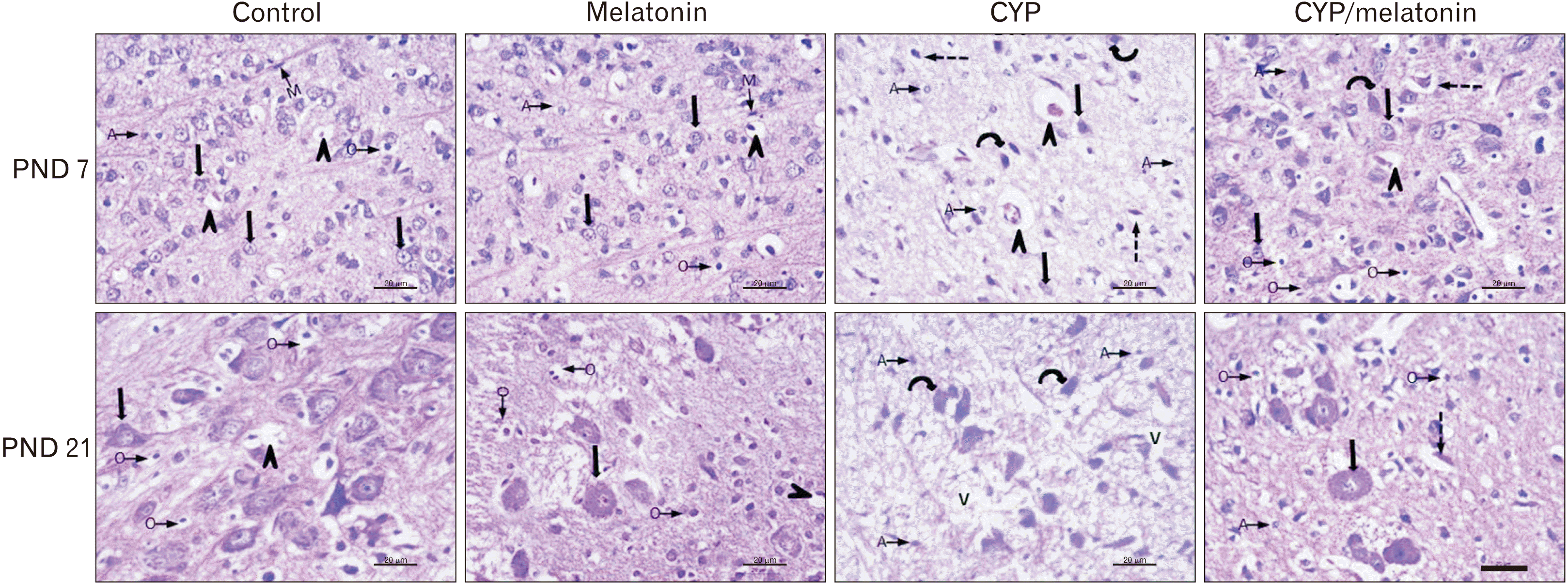 | Fig. 1Representative H&E staining of rat medulla oblongata of control and melatonin aged 7 days: showing medulla neurons appeared small in size, varied in shape and had round nuclei (black arrows). The neuropil contains different neuroglia (O, A, and M) and blood vessels (arrowheads). Medulla oblongata of CYP treated group of the same age shows neurons with central chromatolysis (curved arrows), degenerated neurons and pyknotic nuclei (dashed arrows), dilated blood vessels (black arrowheads) and numerous A. CYP/melatonin treated group resulted in almost normal appearing neuronal cells, except for chromatolysis (curved arrow) and degenerated neurons and pyknotic nuclei (dashed arrow) in some neurons. Increased number of O and deceased of A. Control and melatonin groups aged 21 days, the neurons appear larger (black arrow). Many oligodendrocytes (arrows with rounded end) are observed in the neuropil. CYP treated group show chromatolysis of almost all neurons (curved arrows), a large number of A and v. Co-administration of CYP/melatonin shows more or less normal neurons (black arrow). However, some degenerated cells (curved arrow) with loss of nuclear details could be detected. Few A and more O could be observed. A, astrocyte; CYP, cypermethrin; M, microglia; O, oligodendrocyte; PND, postnatal day; v, vacuolated neuropil. Scale bar=20 μm. |
Semithin sections finding
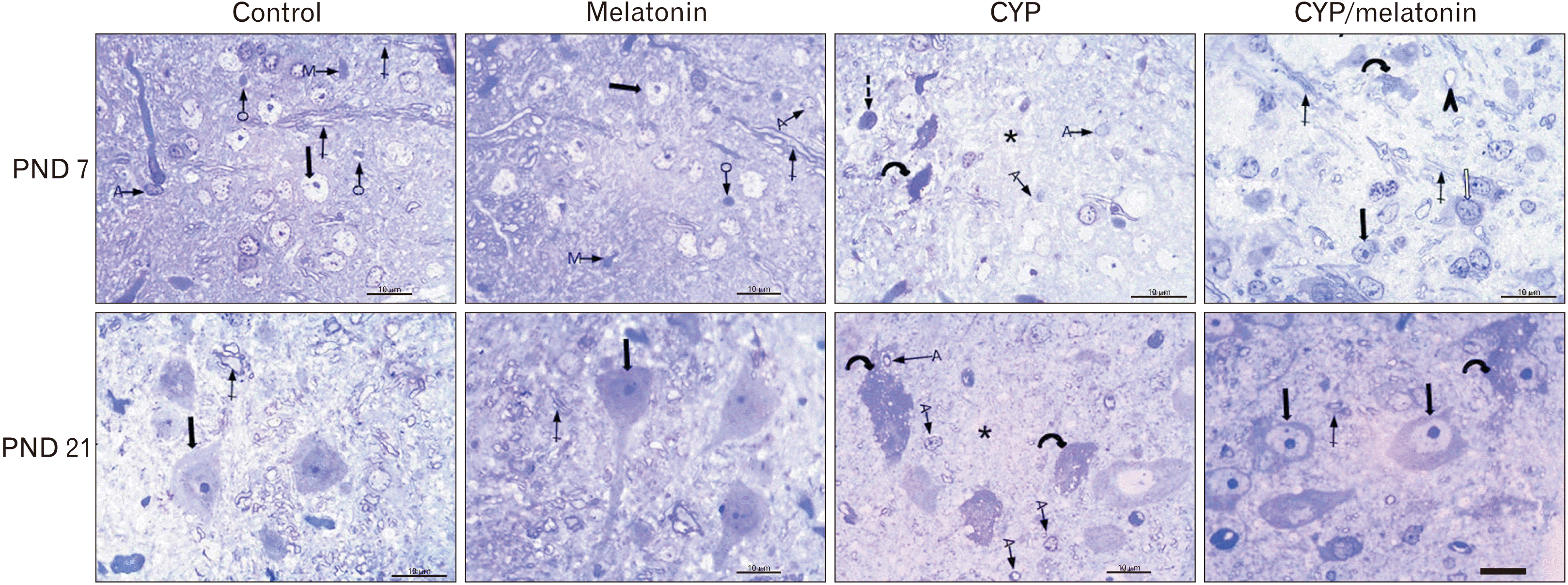 | Fig. 2Representative semi-thin blue-stained sections of the rat medulla oblongata of control and melatonin aged 7 days: showing normal neurons with vesicular nuclei and prominent nucleoli (black arrow), different neuroglia (A, O, and M) and myelinated axons (crossed arrows). CYP treated group of the same age showing some shrunken neuronal bodies with an irregular outline, hardly identified nuclei, and deeply stained vacuolated cytoplasm (dashed arrow). Some neurons appear to have deeply stained nuclei and cytoplasm (curved arrow)., Numerous fine vacuoles were detected in neurons, numerous A, and neuropil devoid of myelinated axons (star). CYP/melatonin treated group shows nearly normal neurons with prominent nucleoli (black arrow), neurons containing coarse clumps of heterochromatin (white arrow), myelinated axons (crossed arrow). Darkly stained neurons (curved arrow) and dilated blood vessels are still observed. Sections of the control and melatonin groups aged 21 days showing large multipolar neurons with large nucleus & prominent nucleolus (black arrow). The neuron is surrounded by dense neuropil containing more myelinated axons (crossed arrow). CYP treated group of the same age shows neurons with numerous fine vacuoles (curved arrows), a large number of A, and neuropil devoid of myelinated axons (star). CYP/melatonin treated group shows neurons with pale vesicular nuclei with prominent nucleoli (black arrows) and myelinated axons in the surrounding neuropil. However, few darkly stained vacuolated neurons are detected. A, astrocyte; CYP, cypermethrin; M, microglia; O, oligodendrocyte; PND, postnatal day. Scale bar=10 μm. |
Ultrastructure finding
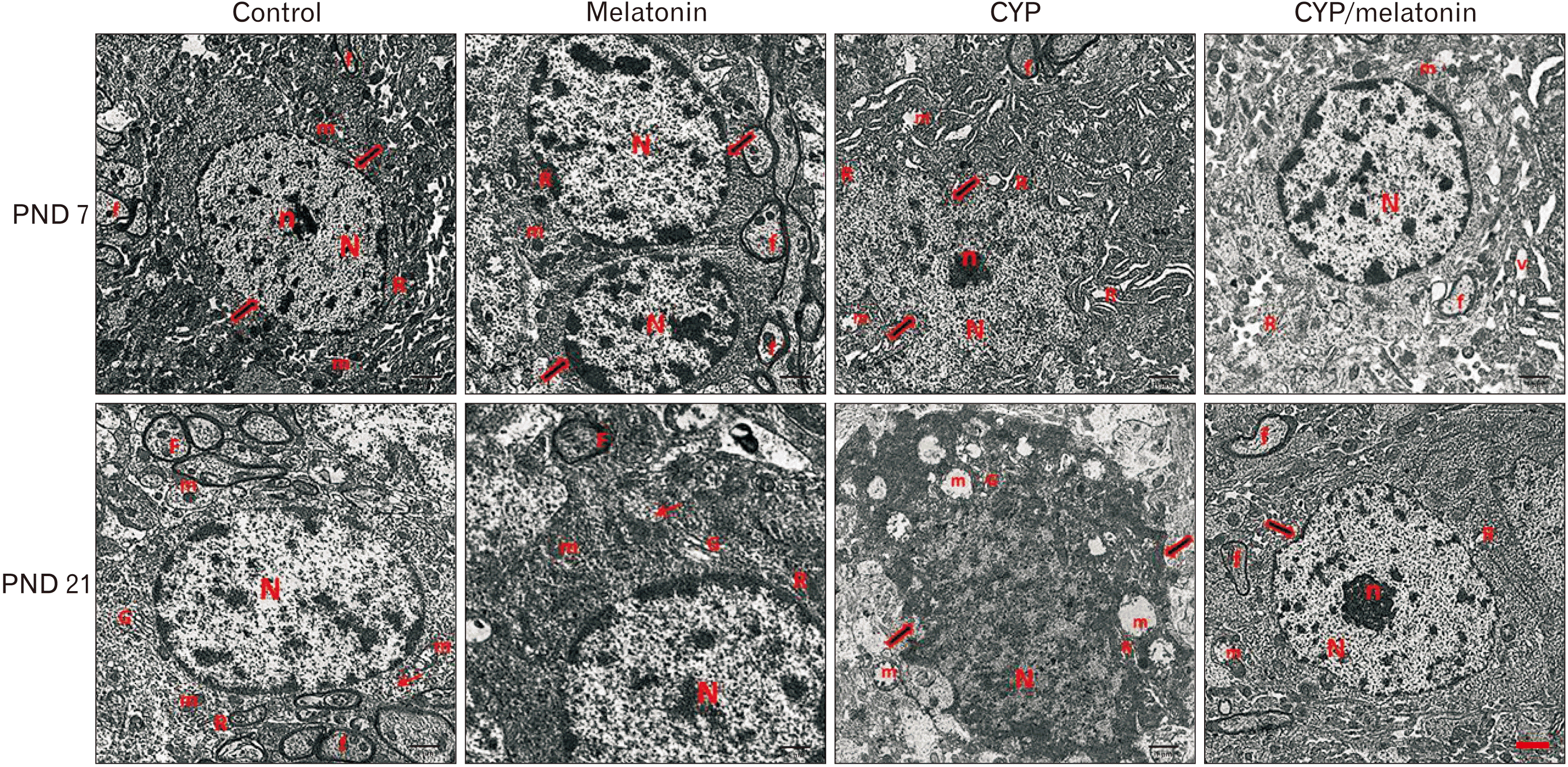 | Fig. 3An electron micrograph of a section in control and melatonin rat medulla oblongata aged 7 days shows motor neurons having rounded euchromatic N with regular nuclear membranes (arrow) and prominent Nu. The homogenous cytoplasm contains normal m and rough endoplasmic R. Myelinated f with preserved myelin sheath are also observed. CYP treated group of the same age displays an irregular N surrounded by an indented nuclear membrane (arrows). The cytoplasm contains dilated cisternae of rER (R) and dilated m with destructed cristae (m). Few myelinated f could be detected. CYP/melatonin treated group shows an apparently normal nerve cell with a regular N but the N still shows heterochromatin. The cytoplasm contains normal more or less normal RER (R) and m. However, a small area of V could be still detected in the cytoplasm. A section of the control and melatonin group aged 21 days shows a large neuron with a large N, the chromatin inside the N is euchromatin mainly and less amount of heterochromatin. The cytoplasm of the neuron contains large m, G, and multiple free ribosomes (arrow). The neuron is surrounded by many myelinated axons (f). CYP treated group of the same age displays markedly affected neurons. The N is shrunken, darkly electron-dense, and irregular in shape with indentation of its nuclear membrane (arrows). Many dispersed irregularly shaped chromatin aggregates are demonstrated throughout the N. The cell has an irregular outline with a shrinkage cytoplasm containing multiple ballooned m with disrupted cristae and dilated rER (R) and G. Medulla oblongata sections of CYP/melatonin treated group of the same age exhibit a euchromatic N, fine dispersed chromatin, and prominent n. More or less normal rER (R) and myelinated axons (f) could be detected. However, mild irregular N (arrow) and ballooned m are still detected (transmission electron microscope, ×17,500, Scale bar=1 μm). CYP, cypermethrin; f, fibers; G, Golgi apparatus; m, mitochondria; N, nucleus; n, nucleolus; Nu, nucleoli; PND, postnatal day; R, reticulum; V, vacuolation. |
Immunohistochemical staining
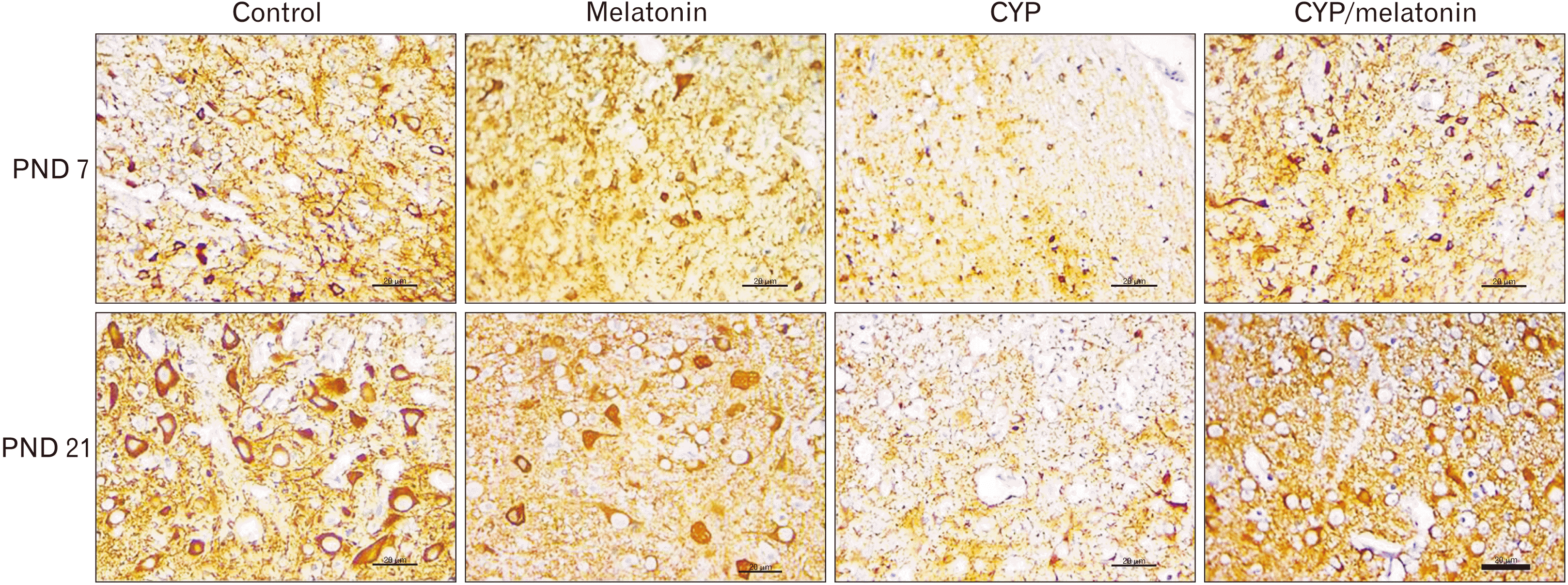 | Fig. 4Expression of MAP-2 in the medulla oblongata of the control, melatonin, CYP, CYP/melatonin groups at 7 and 21 PNDs. CYP administration dramatically decreased the expression of MAP-2 in rat medulla oblongata compared to the control group. Melatonin co-treatment significantly upregulated the expression of MAP-2. CYP, cypermethrin; MAP-2, microtubule-associated protein-2; PND, postnatal day. Scale bar=20 µm. |
 | Fig. 5Area percentage of (A) MAP-2, (B) MBP and (C) GFAP and (D) Olig2+ve cells in the medulla oblongata of the control, melatonin, CYP, CYP/melatonin groups at 7 and 21 PNDs. CYP, cypermethrin; GFAP, glial fibrillary acidic protein; MAP-2, microtubule-associated protein-2; MBP, myelin basic protein; Olig2, oligodendrocyte transcription factor; PND, postnatal day. **P<0.001, compared with the control group; and #P<0.05 and ##P<0.001, compared with the CYP group. data are expressed as means±standard deviation. |
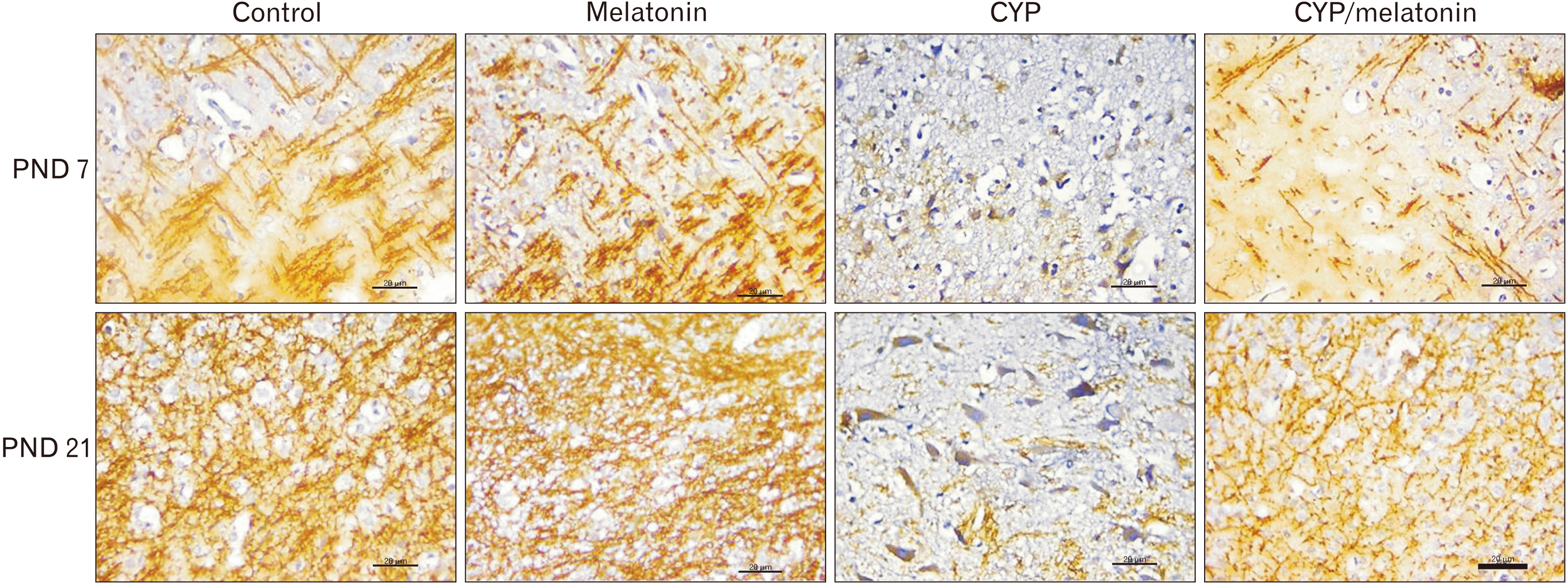 | Fig. 6Expression of MBP in the medulla oblongata of the control, melatonin, CYP, CYP/melatonin groups at 7 and 21 PNDs. CYP administration dramatically downregulated the expression of MBP in rat medulla oblongata compared to the control group. Melatonin co-treatment significantly increased the expression of MBP. CYP, cypermethrin; MBP, myelin basic protein; PND, postnatal day. Scale bar=20 µm. |
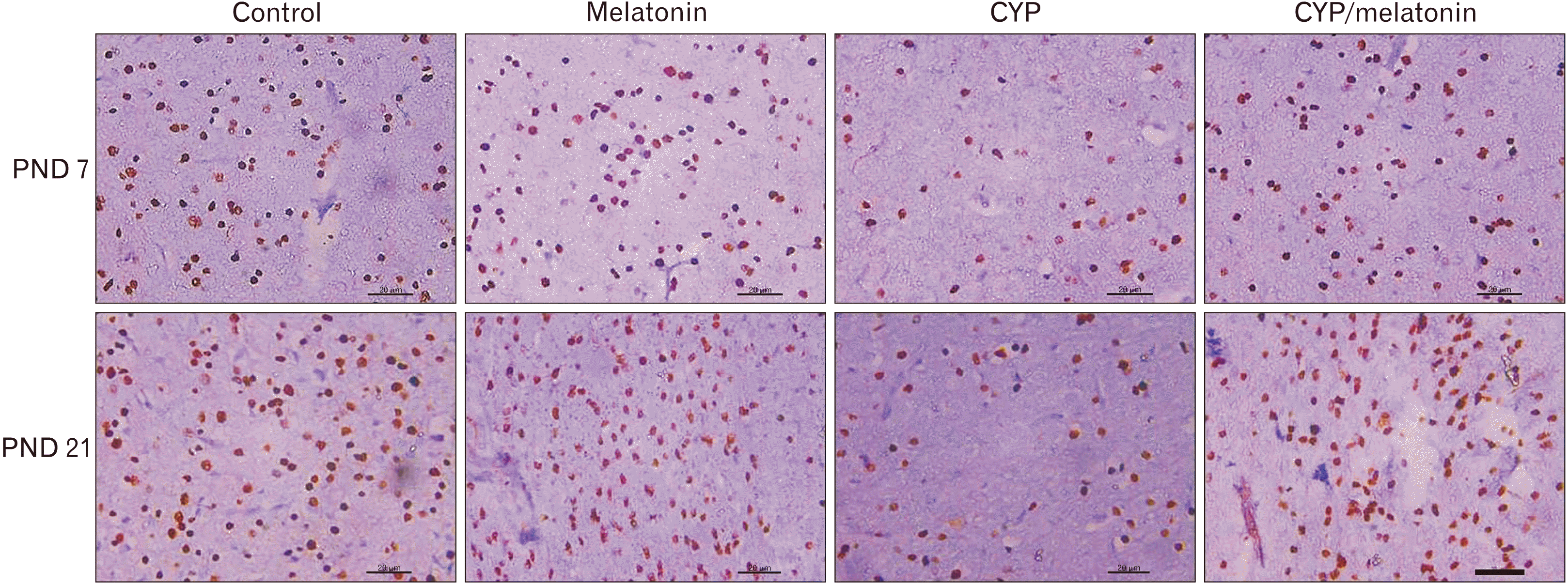 | Fig. 7Expression of Olig2 in the medulla oblongata of the control, melatonin, CYP, CYP/melatonin groups at 7 and 21 PNDs. CYP administration showed a reduction in the expression of Olig2+ve cells in rat medulla oblongata compared to the control group. Melatonin co-treatment increased the expression of Olig2+ve cells. CYP, cypermethrin; Olig2, oligodendrocyte transcription factor; PND, postnatal day. Scale bar=20 µm. |
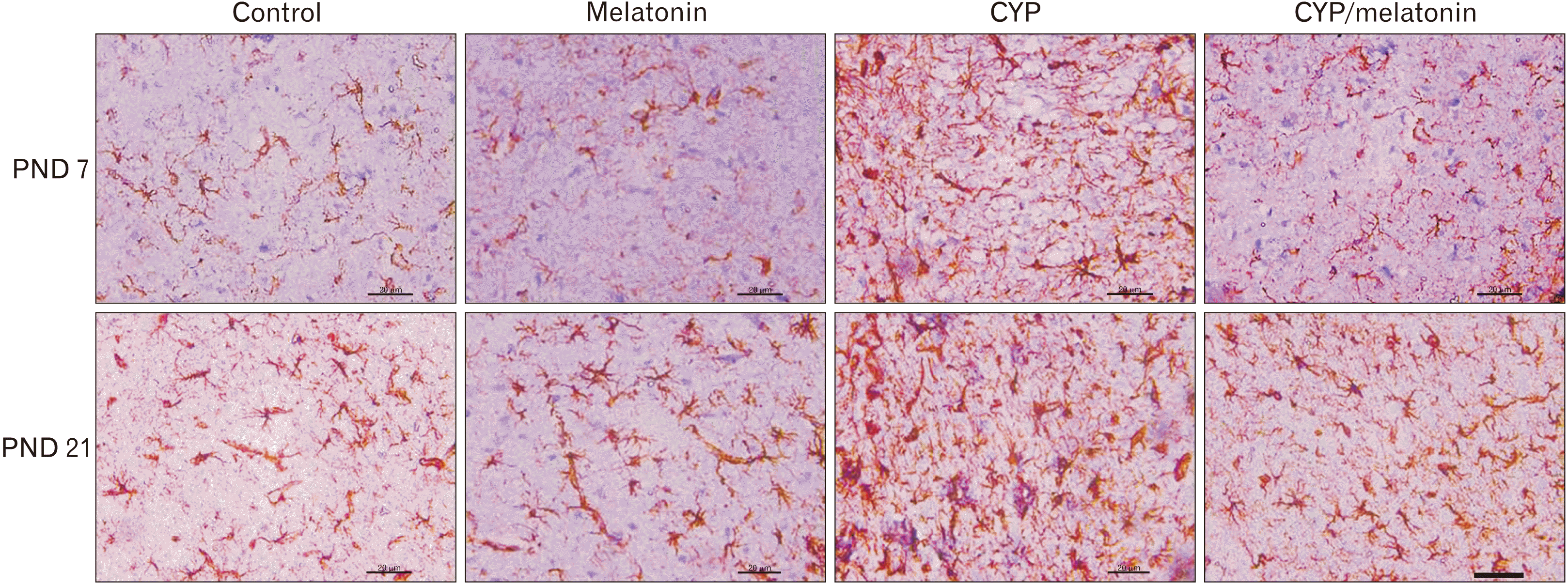 | Fig. 8Expression of GFAP in the medulla oblongata of the control, melatonin, CYP, CYP/melatonin groups at 7 and 21 PNDs. The administration of CYP showed an increase in the expression of GFAP in rat medulla oblongata compared to the control group. Melatonin treatment with CYP reduced the expression of GFAP. CYP, cypermethrin; GFAP, glial fibrillary acidic protein; PND, postnatal day. Scale bar=20 µm. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download