Introduction
The clinical implications associated with several neurovascular structures at the medial side of the ankle such as nerve entrapment and impaired blood circulation. The tarsal tunnel (TT) is the important structures which house the posterior tibial nerve (PTN) and the posterior tibial artery (PTA) including the associated branches of nerve and artery. The medial wall of TT composed of the talus, calcaneus, distal tibia, medial malleolus. The flexor retinaculum forms the TT’s roof. The TT is further separated into medial and lateral tunnels by a fibrous septum. The medial tunnel houses the medial plantar nerve (MPN) and artery, and the lateral tunnel houses the lateral plantar nerve (LPN) and artery [
1]. There are numerous anatomical studies of the neurovascular structures at the medial of the ankle including identification of its variations. The PTN bifurcates into the two plantar nerves that are the medial and LPNs. The bifurcation point of the PTN varies, such as within, proximal, or distal to the TT [
2-
4]. Heel pain is the most common symptom that involves TT syndrome or heel spur [
2,
3,
5,
6]. Moreover, some researchers represent heel pain may be involved medial and inferior calcaneal nerves (ICNs) [
5,
7,
8]. The medial calcaneal nerve (MCN) branches from the PTN and its branches. The MCN has a highly variable origin and a highly variable number of branches. Its origin may be from the PTN, LPN, and MPN [
3,
9,
10]. The number of branch ranges from one to five branches [
9-
12]. For the ICN, it can be called several names such as Baxter’s nerve [
13], the first branch of the LPN, deep calcaneal nerve, and nerve to the abductor digiti minimi (ADM) muscle [
14]. It originates from the LPN [
12]. The number of branches from this nerve is a single to two branches, where a single branching pattern is the most common variation [
10-
12]. The knowledge of the medial and ICNs at the medial heel region is essential for the diagnosis and treatment of heel pain and TT syndrome [
15,
16].
The vascular structures at the medial ankle have a similar pattern with the nerves. The PTA bifurcates into medial and lateral plantar arteries which are distal to the PTN [
2]. However, the medial calcaneal artery (MCA) may originate from branches of the PTA. There are relatively few studies on the branching pattern of these arteries [
17]. Then, the conditions involved with the MCA and inferior calcaneal artery (ICA) such as TT syndrome, calcaneal nail, operative procedure around the ankle [
18] including the injuries of the main branch of these arteries. If the MCA, inferior artery, and their main branches were injured from these conditions, the related structures may be affected. Hence, the anatomical knowledge of vascular structures is essential for clinicians to treat patients.
The neurovascular structures at the medial side of the ankle have more variation, however, further knowledge is still needed. This knowledge is important for clinicians to apply for the diagnosis and treatment of patients at the medial side of the ankle. The lack of anatomical understanding can cause the failure of the treatment [
19]. Hence, the purpose of this study was to describe the branching pattern of neurovascular structures of the medial ankle which comprise of the branching point, bifurcation type, origin, and number of a neurovascular structures.
Go to :

Discussion
There were several studies on the neurovascular structures branching pattern at the medial side of the ankle [
2-
4,
10,
18,
20-
28]. The researchers studied these structures related to the TT and its clinical implications [
2-
4,
10,
18,
20-
23]. The location, branching point, and branching pattern of the neurovascular structures were essential for the management of several clinical conditions. The clinical conditions related to the neurovascular structures at the medial side of the ankle such as TT syndrome [
29-
31], heel pain [
15,
16], the atrophy of ADM muscle [
32], and avascular necrosis of the talus [
33-
35]. The treatment of each condition depended on the knowledge of the neurovascular structures for successful treatment. Furthermore, the anatomical variations of neurovascular structures had differences in each population.
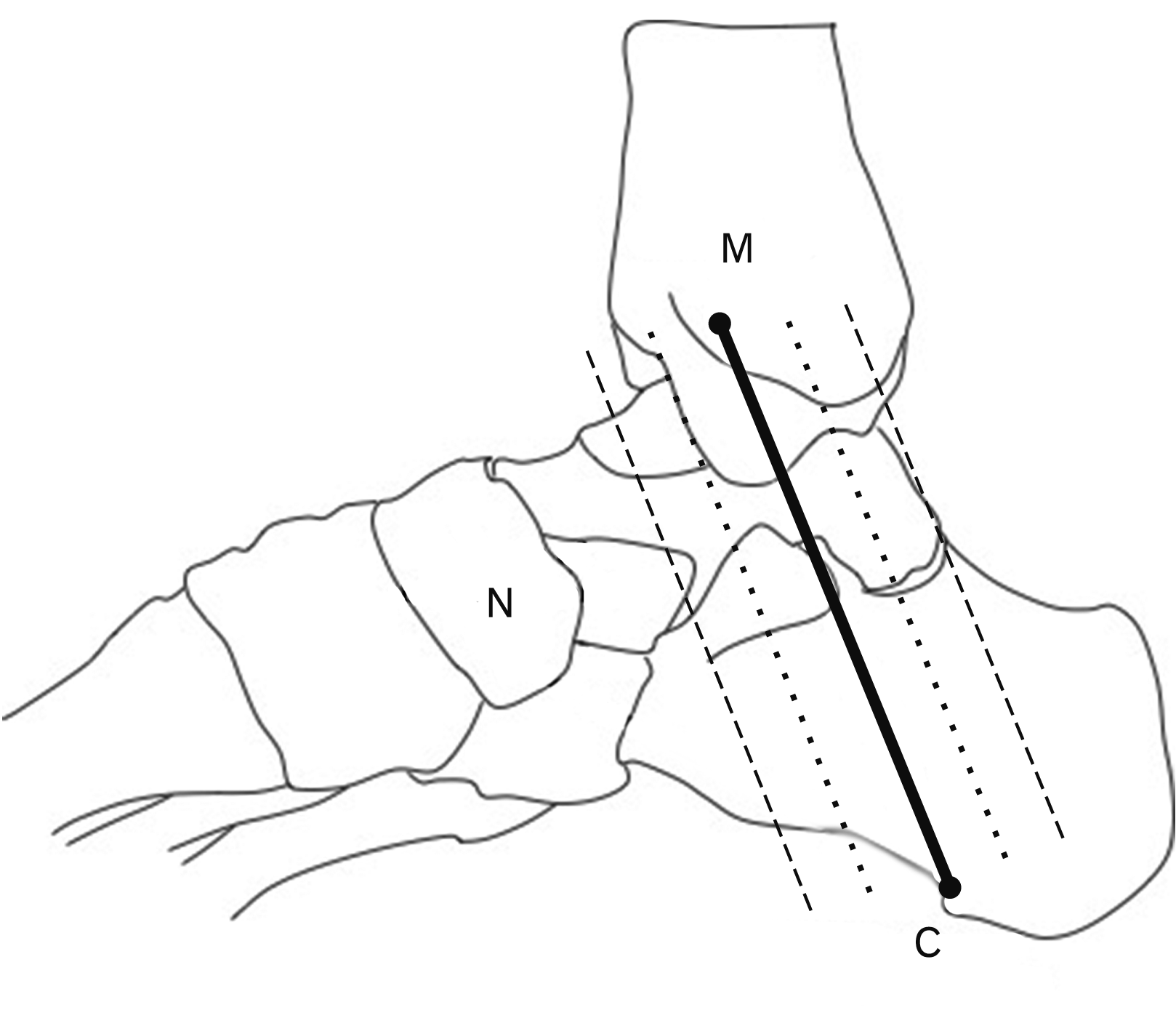
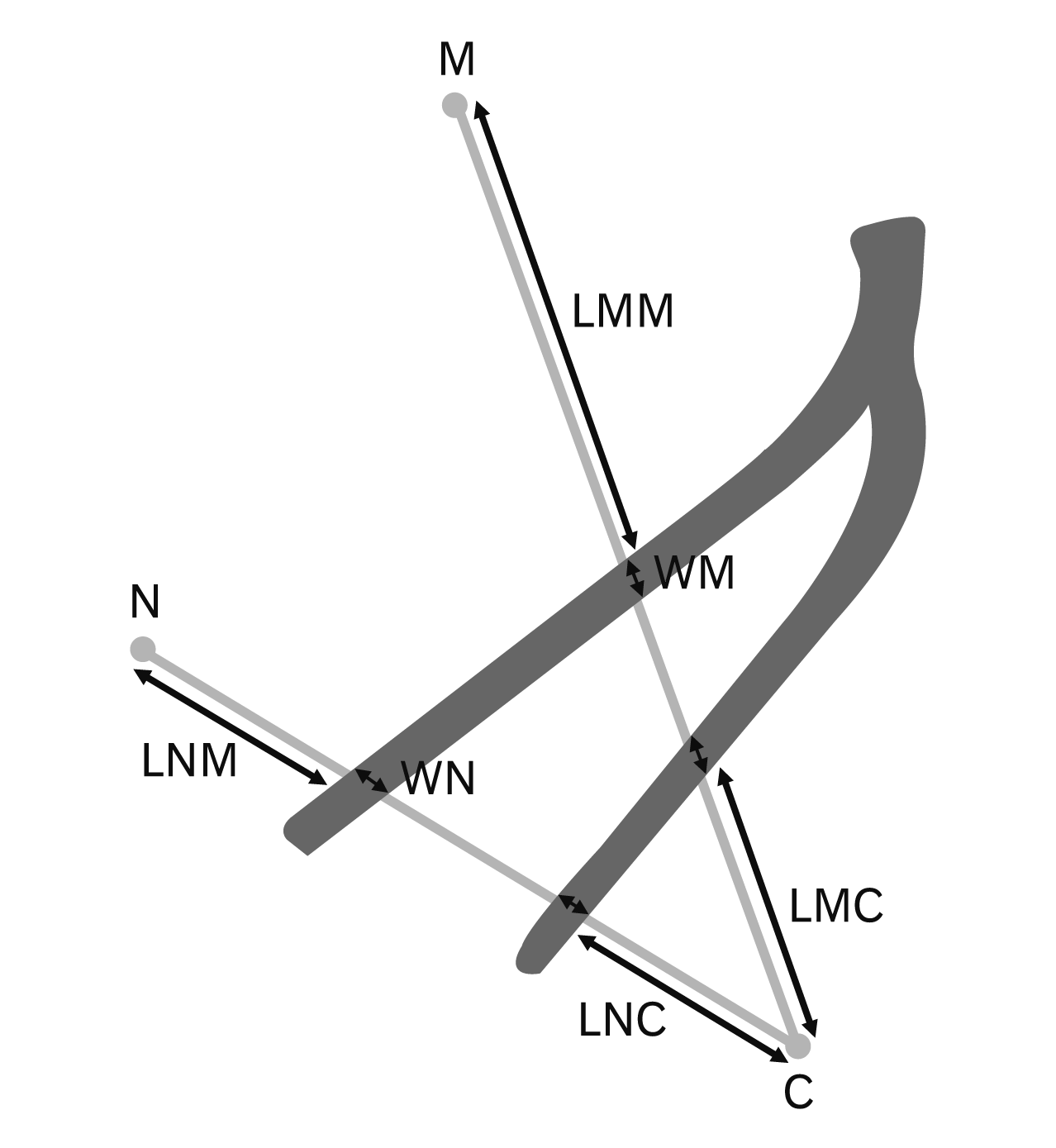
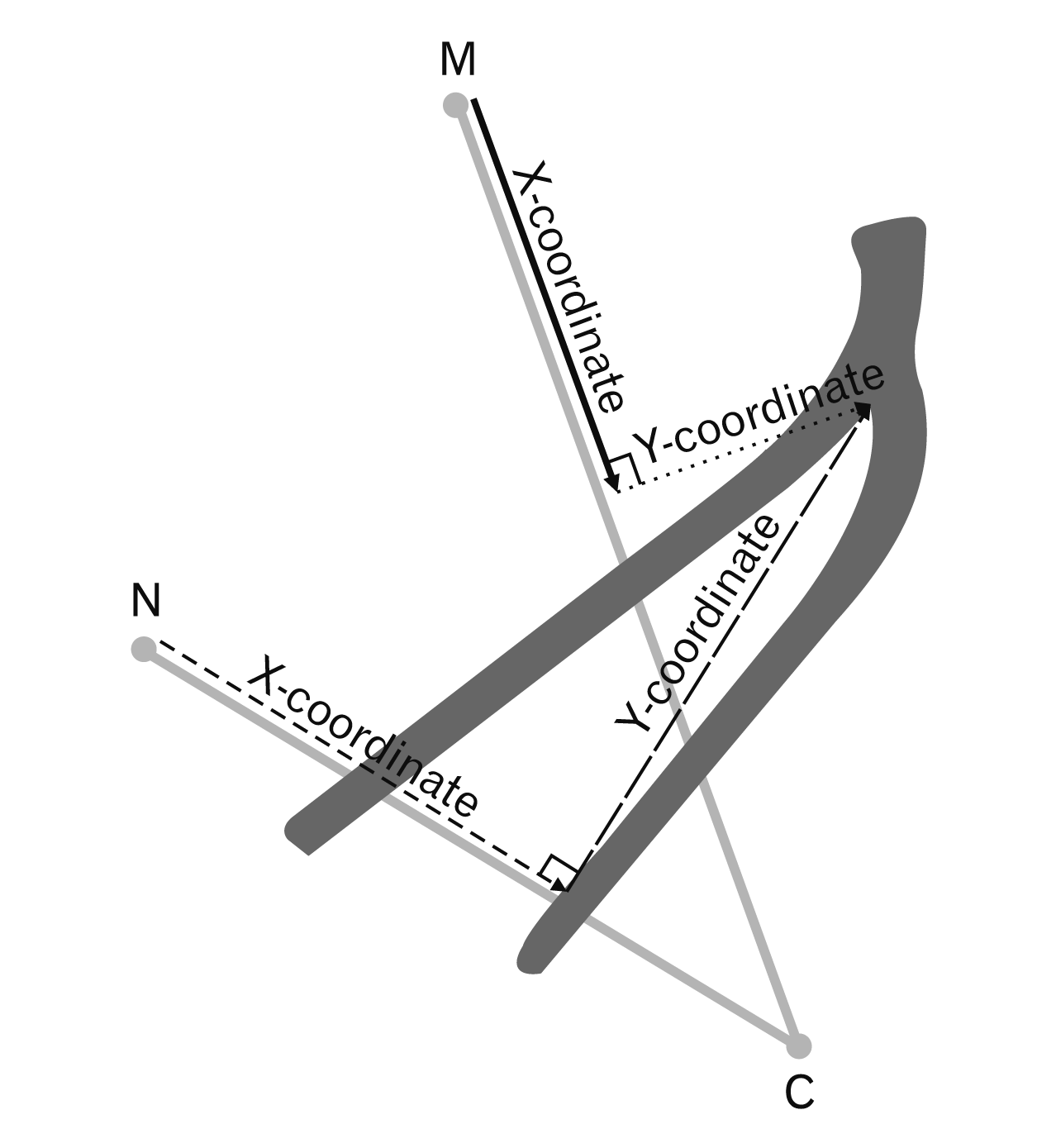
The previous studies relate the location of the plantar nerves to the MC and NC axes [
4,
36]. Heimkes and colleagues [
36] and Bilge and colleagues [
4] measured the location of the MPN from medial malleolus and the location of the LPN from the medial tubercle of the calcaneus. They represented that the safe area for neurovascular structures approximated 20 to 25 mm from medial malleolus and 25 to 30 mm from the medial tubercle of the calcaneus. Moreover, Heimkes and colleagues [
36] measured the location of the MPN from navicular tubercle and the location of the LPN from the medial tubercle of the calcaneus on the NC axis. The safe distances for neurovascular structures approximated 15 mm from medial malleolus and 30 mm from the medial tubercle of the calcaneus. In this study, the areas approximated 35 mm from medial malleolus and 20 mm from the medial tubercle of the calcaneus on the MC axis may be safe for clinical procedures and will not damage the neurovascular structures. For the NC axis, the safe distances for neurovascular structures approximated 25 mm from medial malleolus and 20 mm from the medial tubercle of the calcaneus. The safe areas for neurovascular structures in this study differed from the previous studies [
4,
36] because the MPA and LPA were measured in this study. The information could be applied to the surgical approach about the safe areas for pin insertion.
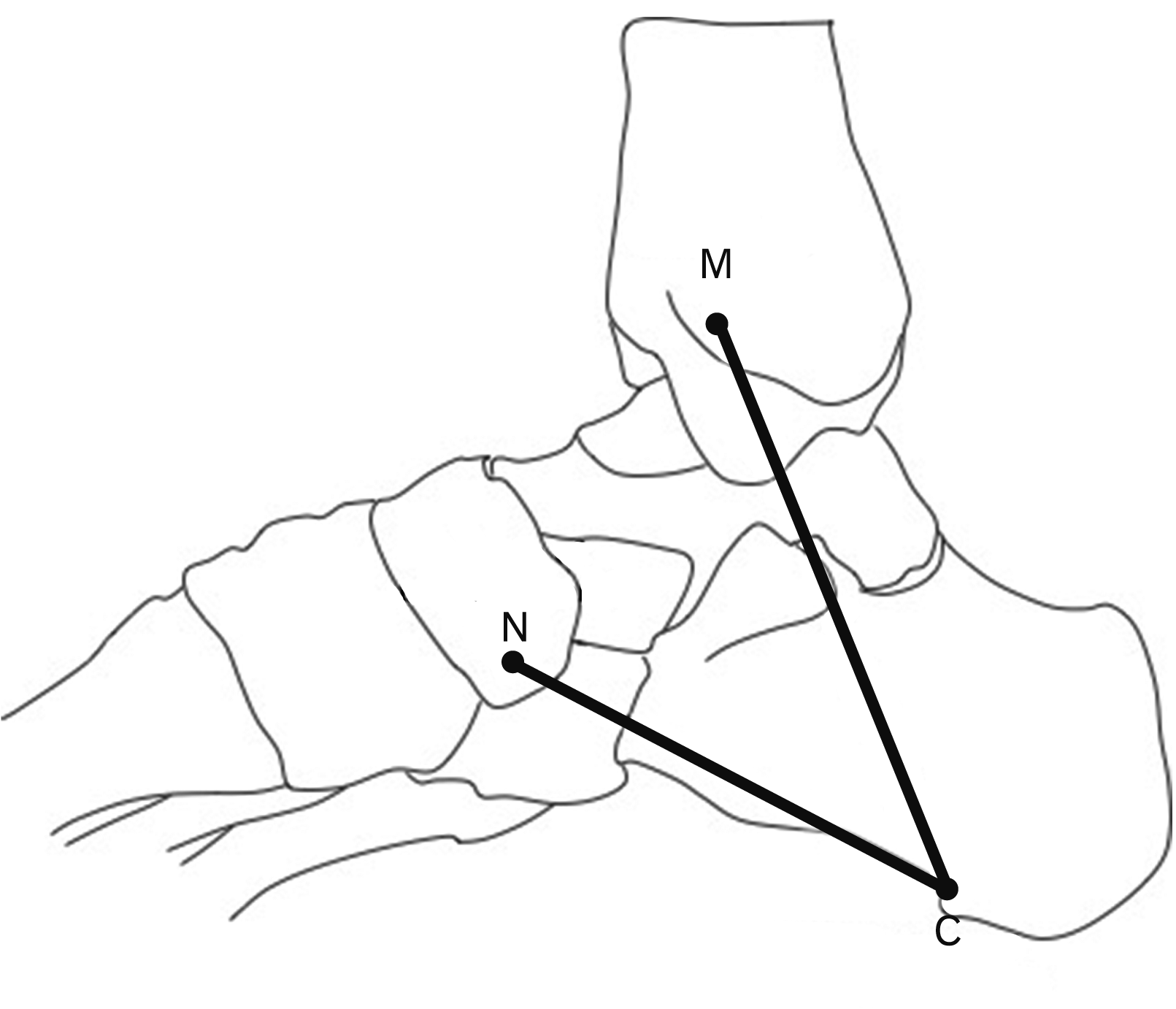
The PTN or tibial nerve in one of the branches of the sciatic nerve [
3]. The PTN divided into two main branches such as MPN and LPN including the MCN and ICN. The bifurcation of PTN in the consensus of the previous researchers occurred in the TT which was different from the current study. The bifurcation point of the PTN in this study bifurcated superior and without the tarsal tunnel (
Table 3). Furthermore, the bifurcation type of PTN in this study based on the classification of Torres and colleagues [
20]. The types of branching points in this study were different from the previous studies (
Table 5) [
2-
4,
18,
20,
22,
24]. These findings of the different types of bifurcation of PTN were important. The high division of the PTN in this study may be related to the development of the TT syndrome in the patients [
2]. In addition, the race and/or the individual difference related to the different findings in this study. Clinically, the TT syndrome could be caused by the neurovascular entrapment in the TT. The compression of the neurovascular structures could be caused by the excessive tension of the flexor retinaculum, bone deformities surrounding the TT, bleeding, and adhesions caused by trauma, neuroma, space-occupying lesions, the placement of the foot in inversion and eversion positions [
29-
31]. The data of these structures, PTN, PTA, and their branches, might improve the anatomical knowledge of neurovascular structures. The branching point of PTN and PTA and the location of their branches might be used as a guideline for the diagnosis and treatment of some diseases of the tarsal region [
15,
16,
37,
38]. The knowledge of the PTN’s division level is important for the diagnosis and treatment of the patient of TT syndrome and numerous surgical procedures. Moreover, this knowledge could be applied in a PTN block which should be injected superiorly to the axis.
Table 5
Branching pattern of PTN and PTA
|
Authors |
Year |
No. |
Branching pattern of PTN |
|
Branching pattern of PTA |
|
|
|
Type I |
Type II |
Type III |
Type IV |
Type V |
Type I |
Type II |
Type III |
Type IV |
Type V |
|
Dellon and Mackinnon [2] |
1984 |
31 |
22.58% |
54.84% |
16.13% |
6.45% |
|
|
|
|
|
|
|
|
Heimkes et al. [36] |
1987 |
60 |
100% |
|
|
|
|
|
|
|
|
|
|
|
Havel et al. [3] |
1988 |
68 |
45.59% |
38.24% |
8.82% |
7.35% |
|
|
|
|
|
|
|
|
Bilge et al. [4] |
2003 |
50 |
84% |
12% |
|
|
4% |
|
10% |
44% |
|
|
46% |
|
Torres and Ferreira [20] |
2012 |
50 |
52% |
14% |
22% |
12% |
|
|
|
|
|
|
|
|
Malar [24] |
2016 |
20 |
85% |
10% |
5% |
|
|
|
|
|
|
|
|
|
Tamang et al. [23] |
2016 |
30 |
90% |
10% |
|
|
|
|
|
93.3% |
|
|
6.6% |
|
Yang et al.a) [18] |
2017 |
60 |
70% |
7% |
|
18% |
|
|
71.7% |
28.3% |
|
|
|
|
Iborra et al. [22] |
2018 |
12 |
91.7% |
|
|
8.3% |
|
|
|
|
|
|
|
|
Present study |
2020 |
40 |
50% |
|
|
50% |
|
|
97.5% |
|
2.5% |
|
|

The knowledge of the MCN was important for the diagnosis and treatment of heel pain [
15,
16]. Typically, the MCN was one of the PTN’s branches. The origin of MCN in the consensus of the previous researchers was similar to the current study (
Table 6) [
2,
3,
9-
12,
18,
20,
21,
23,
24,
39,
40]. Besides, the branching point of the MCN was highly variable. The MCN could bifurcate proximal to the TT, within the TT, or distal to the TT [
2,
3,
9-
12,
18,
20,
21,
23,
24,
39,
40]. These findings from the previous and current studies were important for the symptoms of the patient. The proximal branch of the MCN ran outside of the TT to the medial side of the heel and the calcaneus [
41]. It involved the jogger’s heel which was the compression syndrome that caused heel pain [
42,
43]. Moreover, the MCN in the patient with the TT syndrome in 35% to 40% might be spared. Within the TT, the MCN was compressed which involved the localized symptoms at the heel. Prolonged standing and walking may be worsened symptoms. The rest and removal of the shoes improved these symptoms [
44]. The number of branches of MCN type in this study differed from all previous studies which were seen in
Table 6 [
2,
3,
9-
12,
18,
20,
21,
23,
24,
39,
40]. In addition, our research found 28 types of branch patterns of the MCN. Branching patterns of MCN in this study were similar and different from MCN’s pattern of one previous study [
20]. Our study was similar to the first pattern (two cases), second pattern (four cases), third pattern (one case), forth pattern (one case), seventh pattern (four cases), eleventh pattern (two cases), and twelfth pattern (two cases). The current study differed from the previous study because we had a high branching point of PTN. Therefore, the branching pattern had numerous patterns than the previous study.
Table 6
The studies of origin and number of branching of MCN
|
Authors |
Year |
No. |
Type of sample |
Origin of MCN |
|
Number of branching |
|
|
|
PTN |
Bifurcation of PTN |
LPN |
MPN |
2 nerves |
>2 nerves |
1 |
2 |
3 |
4 |
5 |
|
Dellon and Mackinnon [2] |
1984 |
20 |
Patients |
18 |
|
2 |
|
|
|
|
15 |
5 |
|
|
|
|
Havel et al. [3] |
1988 |
68 |
Embalmed |
47 |
|
13 |
1 |
8 |
|
|
54 |
14 (>1 branch) |
|
|
Davis and Schon [21] |
1995 |
20 |
Embalmed |
15 |
|
2 |
3 |
|
|
|
8 |
12 (>1 branch) |
|
|
Louisia and Masquelet [10] |
1999 |
15 |
Embalmed (13) |
10 |
|
3 |
|
2 |
|
|
2 |
9 |
2 |
2 |
|
|
Fresh (2) |
|
Dellon et al. [9] |
2002 |
85 |
Patients |
48 |
|
56 |
39 |
|
|
|
31 |
35 |
16 |
3 |
|
|
Govsa et al. [11] |
2006 |
50 |
Embalmed |
11 |
|
7 |
|
15 |
6 |
|
|
1–4 branches |
|
|
Torres and Ferreira [20] |
2012 |
50 |
Fresh |
45 |
|
1 |
4 |
|
|
|
29 |
17 |
4 |
|
|
|
Kim et al. [12] |
2015 |
11 |
Fresh |
7 |
3 |
1 |
|
|
|
|
|
None |
|
|
|
|
Kim et al. [39] |
2015 |
90 |
Embalmed |
24 |
16 |
|
|
50 |
|
|
29 |
38 |
16 |
3 |
1 |
|
Sharma et al. [40] |
2015 |
60 |
Embalmed |
21 |
|
|
|
24 |
15 |
|
21 |
24 |
9 |
5 |
1 |
|
Malar [24] |
2016 |
20 |
Embalmed |
17 |
|
|
|
3 |
|
|
10 |
6 |
4 |
|
|
|
Tamang et al. [23] |
2016 |
30 |
Embalmed |
|
|
17 |
|
13 |
|
|
7 |
16 |
7 |
|
|
|
Yang et al. [18] |
2017 |
60 |
- |
2 |
4 |
29 |
|
22 |
3 |
|
16 |
31 |
10 |
2 |
1 |
|
Present study |
2020 |
40 |
Fresh |
18 |
|
2 |
1 |
16 |
3 |
|
8 |
15 |
17 |
|
|

The knowledge of the ICN at the medial heel region is essential for the diagnosis and treatment of heel pain [
15,
16]. The ICN branched typically from the LPN, however, its origin had variable [
10,
11,
41]. The origin of ICN in the consensus of the previous researchers was similar to the current study (
Table 7) [
10-
12,
20-
22], except for one study. Govsa and colleagues [
11] found that most origins of the ICN were the PTN. Besides, the branching point of the ICN was highly variable. The ICN could bifurcate proximal to the TT, within the TT, or distal to the TT. Most branching point of the ICN in the previous studies was inside the TT [
10,
11,
20,
22] which was similar to the current study. These findings from the previous and current studies were important for the symptoms of the patient. The pain point for compression of the ICN was anterior to the posterior heel approximate four to five cm or distal to the medial tubercle of the calcaneus. The ICN’s compression caused burning pain which radiated to the plantar foot at the lateral side [
6] include the atrophy of ADM muscle [
32]. Moreover, the feeling of pain at the plantar foot led to a change in the position of the patient’s feet during walking [
20]. The number of branches of ICN type in this study differed from all previous studies which were seen in
Table 7 [
10-
12,
20-
22]. In addition, our research found six types of branch patterns of the ICN.
Table 7
The studies of origin and number of branching of ICN
|
Authors |
Year |
No. |
Type of
sample |
Origin of ICN |
|
Number of branching |
|
|
|
PTN |
Bifurcation of PTN |
LPN |
MPN |
2 nerves |
>2 nerves |
1 |
2 |
|
Davis and Schon [21] |
1995 |
20 |
Embalmed |
|
|
20 |
|
|
|
|
|
|
Louisia and Masquelet [10] |
1999 |
15 |
Embalmed (13) |
|
|
14 |
1 |
|
|
|
|
15 |
|
Fresh (2) |
|
Govsa et al. [11] |
2006 |
50 |
Embalmed |
41 |
5 |
2 |
|
2 |
|
|
50 |
|
|
Torres and Ferreira [20] |
2015 |
50 |
Fresh |
9 |
4 |
35 |
|
|
2 |
|
50 |
|
|
Kim et al. [12] |
2015 |
11 |
Fresh |
|
|
11 |
|
|
|
|
11 |
|
|
Iborra et al.a) [22] |
2018 |
12 |
Fresh |
|
|
11 |
|
|
|
|
|
|
|
Present study |
2020 |
40 |
Fresh |
|
|
39 |
|
1 |
|
|
35 |
5 |

The knowledge of the various locations and branching points of these nerves could improve the understanding regarding heel pain especially the medial and inferior site of the heel. Moreover, the calcaneal nerves might be endangered during the calcaneal osteotomy [
4]. This information offers an application advantage for safe incisions, safe screw insertions, external fixation at the calcaneus, diagnosis, and treatment of medial ankle injuries including prevention of neuroma formation during operation. Also, the anatomical pattern of these nerves could improve regional ankle region knowledge for surgical operations.
In this study, we had clarified the location of the branching point of the PTA. From literature, the PTA divided from the popliteal artery at the inferior border of the popliteus muscle [
28]. The PTA accompanied the PTN [
26]. The MPA and LPA bifurcated from the PTA at deep in the abductor halluces muscle. The MPA supplied the instep of the foot, moreover, the LPA supplied the lateral midfoot and forefoot [
41]. The branching point of the PTA in the previous studies bifurcated distal to the PTN [
4,
18,
23,
26] and located within the TT [
18,
23,
45] which were similar to the current study. On the other hand, Bilge and colleagues [
4] and Tamang and colleagues [
23] found that the PTA bifurcated distal to the TT which was not found in this study. Clinically, the complications of the PTA involved avascular necrosis of the talus. The PTA was one of the three extra-osseous arterial contributed branches which supplied the talus. Branches of the PTA supplied medial, inferior, and posterior aspects of the talus [
33-
35]. The PTA and its branches connected with other arteries such as the anterior tibial artery and its branches [
17]. Each area of the talus was supplied with different anastomosis networks [
46]. The injured PTA might be led to the talus necrosis. For the MPA, this artery was important for endovascular recanalization because the MPA branched to the superficial and deep branches which anastomosed with other arteries to supply dorsum of foot or the first toe. In addition, the LPA formed the plantar arch and anastomosed with anterior circulation for supply forefoot [
26].
Researches of the MCA is still limited. This artery ran inferior and branched like a tree to supply the medial heel region [
41]. The study of the number and origin of the MCA was highly variable. The current study had different numbers, origin, and common types from the previous studies which were shown in
Table 8 [
18,
39]. The branching point of the MCA in the previous studies bifurcated distal to the MCN [
18,
39]. The MCA branched superior to the MC axis [
18,
39] which was similar to the current study. Furthermore, our research found 23 types of branch patterns of the MCA which differed from the previous study [
18]. The origin and number of MCA in the current study had differences. Therefore, the branching pattern had several patterns than the previous study. Typically, the MCA was the first branch that bifurcated from the PTA at the ankle level [
28]. Moreover, the MCA was the first branch of the posterior circulation which supplied the medial malleolus region and medial plantar heel [
26]. The causes of injuries involved the MCA such as TT syndrome, calcaneal nail, and operative procedures which might lead to a change in the blood supply of the related region [
18].
Table 8
The studies of origin and number of branching of MCA
|
Authors |
Year |
No. |
Type of
sample |
Origin of MCA |
|
Number of branching |
|
|
|
PTA |
Bifurcation of PTA |
LPA |
MPA |
2
arteries |
>2
arteries |
1 |
2 |
3 |
4 |
5 |
|
Kim et al. [39] |
2015 |
90 |
Embalmed |
23 |
|
39 |
|
28 |
|
|
51 |
28 |
11 |
|
|
|
Yang et al. [18] |
2017 |
60 |
- |
|
|
35 |
|
23 |
2 |
|
6 |
28 |
18 |
8 |
|
|
Present study |
2020 |
40 |
Fresh |
2 |
|
5 |
|
17 |
16 |
|
2 |
7 |
15 |
11 |
5 |

The research of the ICA was also limited. The study of the number and origin of ICA was not cleared. In this study, the ICA bifurcated from the LPA which accompanied the ICN. The ICA bifurcated within the TT closed to the medial process of the calcaneus. Our research identified ten types of branch patterns of the ICA. The complication of the ICA might relate to the injury of the LPA, MPA, PTA, or MCA. The injured ICA might affect the related structures which were supplied from the PTA and its branches.
Clinically, the anatomical knowledge of vascular structures is essential information. The incision in operations must be in the correct location. The blood flow should be located on either side of the incision for optimized healing [
17]. A free flap can be dissected out with high reliability. The vascular knowledge could decrease the risk of distal tissue necrosis in an amputated patient. Moreover, this knowledge advantage for revascularization can increase wound healing or healing of lesions.
This study may have been limited by the small cadavers. Moreover, the shrinkage tissue of the cadaver after death during dissection which may be occurred. Iborra and colleagues [
22] compared the measurements from dissection and the high-resolution US in cadavers showed that the high-resolution US was as effective as the dissection of anatomical variations of the nerves at the medial side of the ankle. The noninvasive technique had a slight error about the number of branches in one specimen. The small diameter of the nerves may not be detected by the high-resolution US. However, the comparison between the invasive and noninvasive measurements could increase the reliability for clinicians to treat the patients. In the future, the small invasive techniques will be more success rate.
In conclusion, the knowledge of the location, width, number, origin, branching point, and branching types of neurovascular structures at the medial side of the ankle could help clinicians to understand the anatomy of these structures. It will benefit for clinicians in making better patient treatment decisions. Hence, the understanding of the anatomy of nerves and vessels in the medial tarsal region is essential for clinicians. The anatomical knowledge of neurovascular structures in the medial tarsal region will be a continued study in the future.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download