Introduction
Materials and Methods
Results
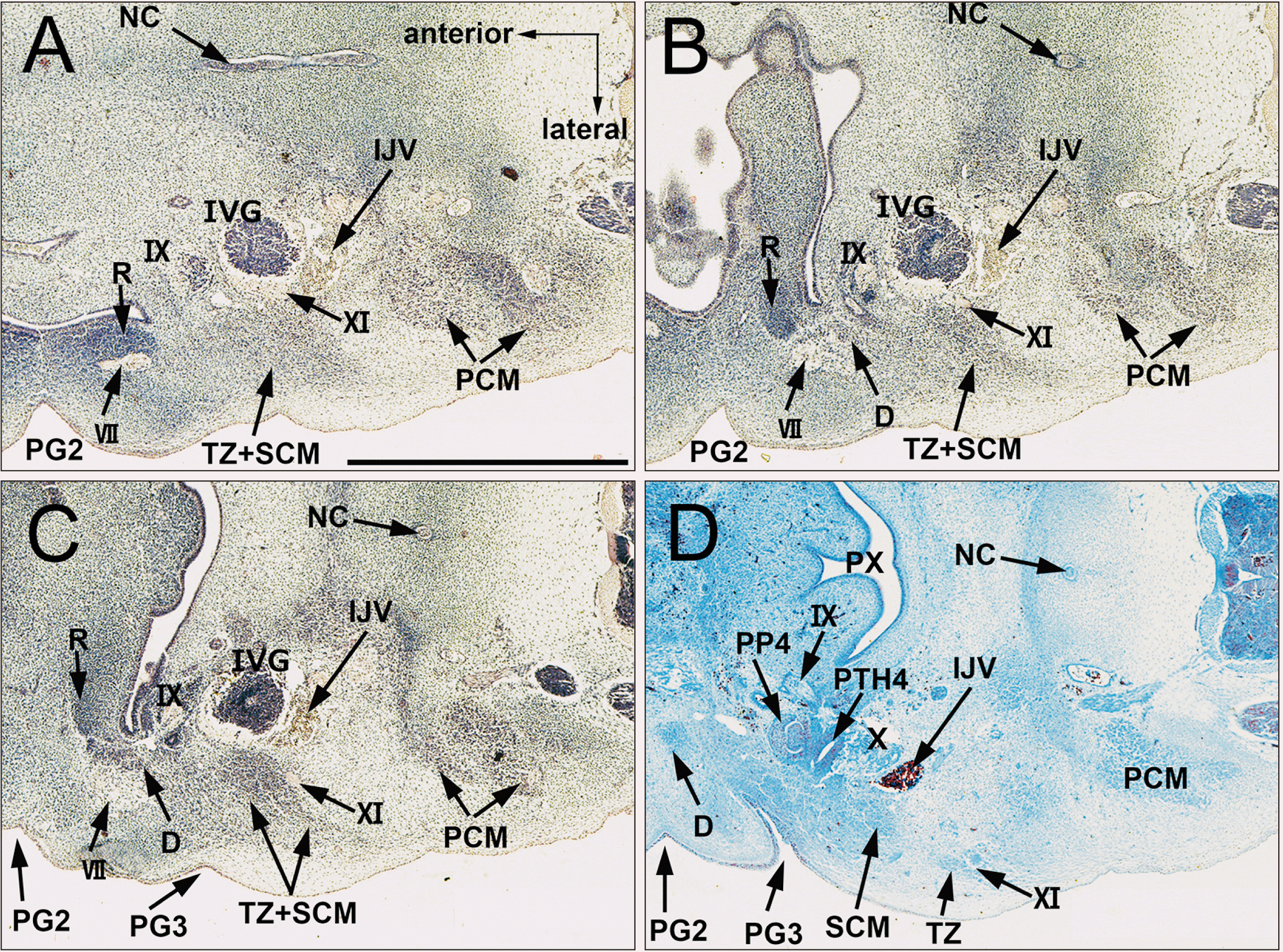 | Fig. 1A single mesenchymal condensation of the SCM and trapezius muscles. CS 16 (6 WD) human embryo (maximum length, 11 mm). Horizontal sections stained with H&E. (A or D) is the most cranial (or caudal) level of the figure. The SCM and trapezius muscles were identified as a single mesenchymal condensation (A–C) located at the lateral side of the nodose ganglion, which started dividing into the two muscles at the inferior end (D). CS, Carnegie stage; D, digastric muscle; IJV, internal jugular vein; IVG: inferior vagal ganglion; IX, glossopharyngeal nerve; NC, notochord; PCM, posterior cervical muscles; PG2, second pharyngeal groove; PG3, third pharyngeal groove; PP4, forth pharyngeal pouch; PTH4, parathyroid IV; PX, pharyux; R, Reichert’s cartilage (second pharyngeal cartilage); SCM, sternocleidomastoid muscle; TZ, trapezius muscle; VII, facial nerve; XI, accessory nerve; WD, weeks of development. |
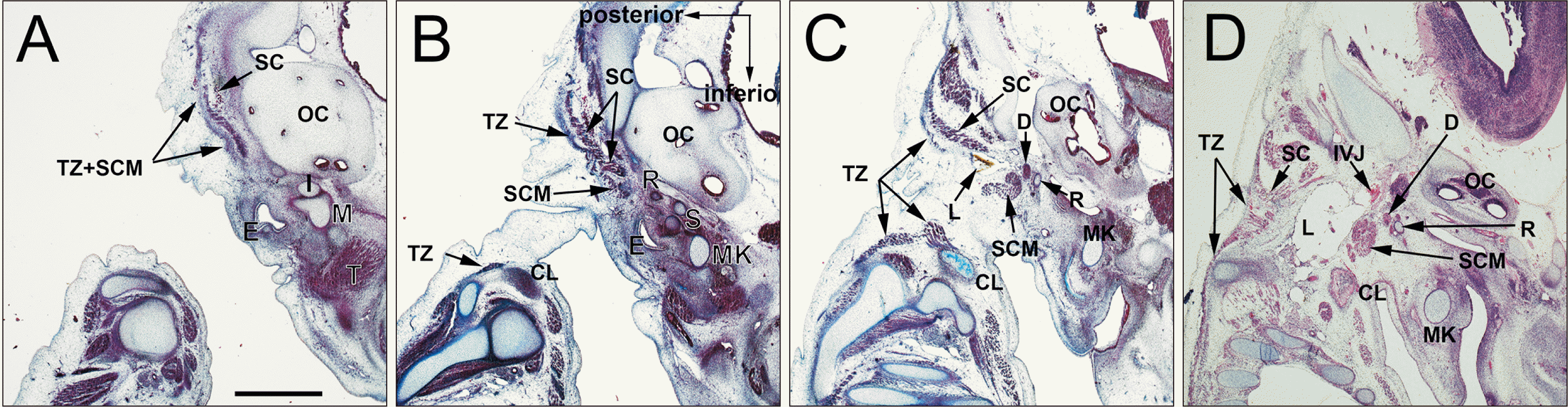 | Fig. 2Differentiation of a single mesenchymal condensation into individual muscles ①. CS 18 (6 WD) human embryo (maximum length, 15 mm). Sagittal sections were stained with H&E. (A or D) is the most lateral (medial) side of the figure. The SCM and trapezius muscles were separated and extended posteriorly, but were connected by a fascia in the upper half (A), while the inferior halves of the two muscles were identified as the anterior and posterior muscle masses, respectively (B, C). The digastricus posterior belly is located between R and the SCM (C, D). CL, clavicle; CS, Carnegie stage; D, digastric muscle; E, external acoustic meatus; I, incus; IJV, internal jugular vein; L, lymphatic vessels; M, mallevs; MK, Meckel’s cartilage (first pharyngeal cartilage); OC, Otic capsule; R, Reichert’s cartilage (second pharyngeal cartilage); S, stapes; SC, splenius capitis muscle; SCM, sternocleidomastoid muscle; T, temporalis; TZ, trapezius muscle; WD, weeks of development. |
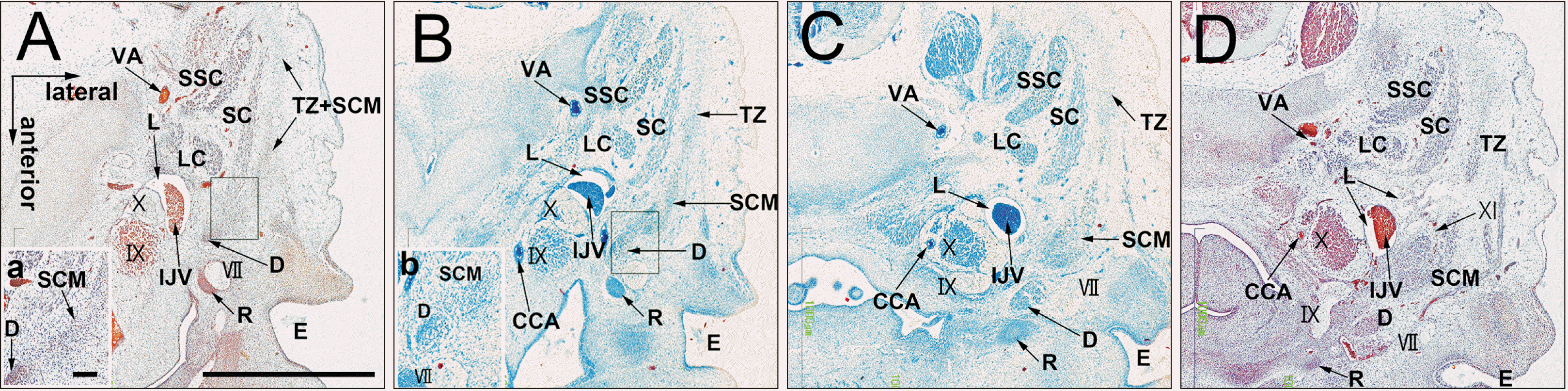 | Fig. 3Differentiation of a single mesenchymal condensation into individual muscles ②. CS 20 (8 WD) human embryo (maximum length, 18 mm). Horizontal sections were stained with H&E. (A or D) is the most cranial (caudal) side of the figure. (a, b) is a high magnification view of the square in (A, B). The SCM and trapezius muscles were separated and extended posteriorly, but connected by a fascia in the upper half. The inferior halves of the two muscles were identified as the anterior and posterior muscle masses, respectively (A–C). The digastricus posterior belly is located between Reichert’s cartilage and the SCM (A–D). The digastricus muscle is close, but not attached, to the SCM (a, b). CCA, common carotid artery; CS, Carnegie stage; D, digastric muscle; E, external acoustic meatus; IJV, internal jugular vein; IX, glossopharyngeal nerve; L, lymphatic vessels; LC, longissimus capitis; R, Reichert’s cartilage (second pharyngeal cartilage); SC, splenius capitis muscle; SCM, sternocleidomastoid muscle; SSC, semispinalis cervicis; TZ, trapezius muscle; WD, weeks of development; X, vagus nerve; VA, vertebral artery; VII, facial nerve. |
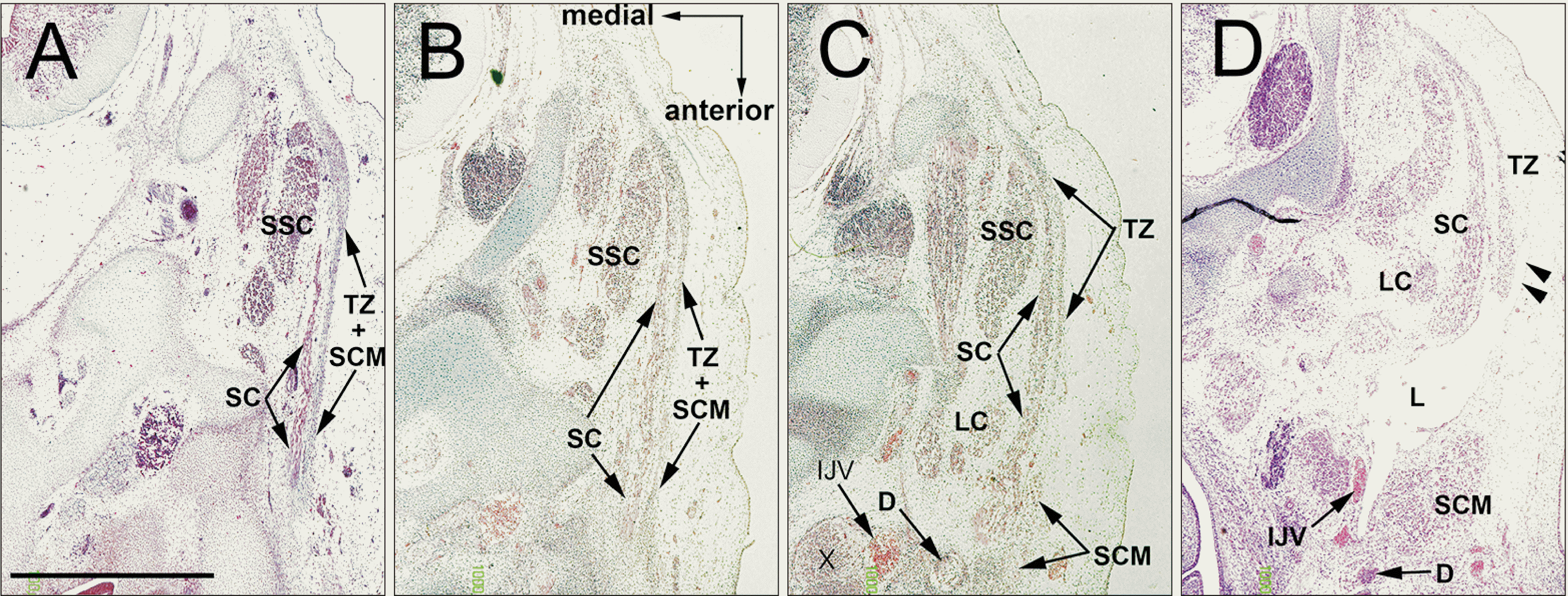 | Fig. 4Lymphatic vessels forming connections with the subcutaneous tissue. CS 20 (8 WD) human embryo (maximum length, 21 mm). Horizontal sections. (A or D) is the most cranial (caudal) side of the figure. The SCM and T extended more posteriorly than in the earlier stages (A–D). The L were dilated and the accompanying afferents formed connections with the subcutaneous tissue through a space between the SCM and TZ (D, arrow heads). CS, Carnegie stage; D, digastric muscle; IJV, internal jugular vein; L, lymphatic vessels; LC, longissimus capitis; SC, splenius capitis muscle; SCM, sternocleidomastoid muscle; SSC, semispinalis cervicis; TZ, trapezius muscle; WD, weeks of development; X, vagus nerve. |
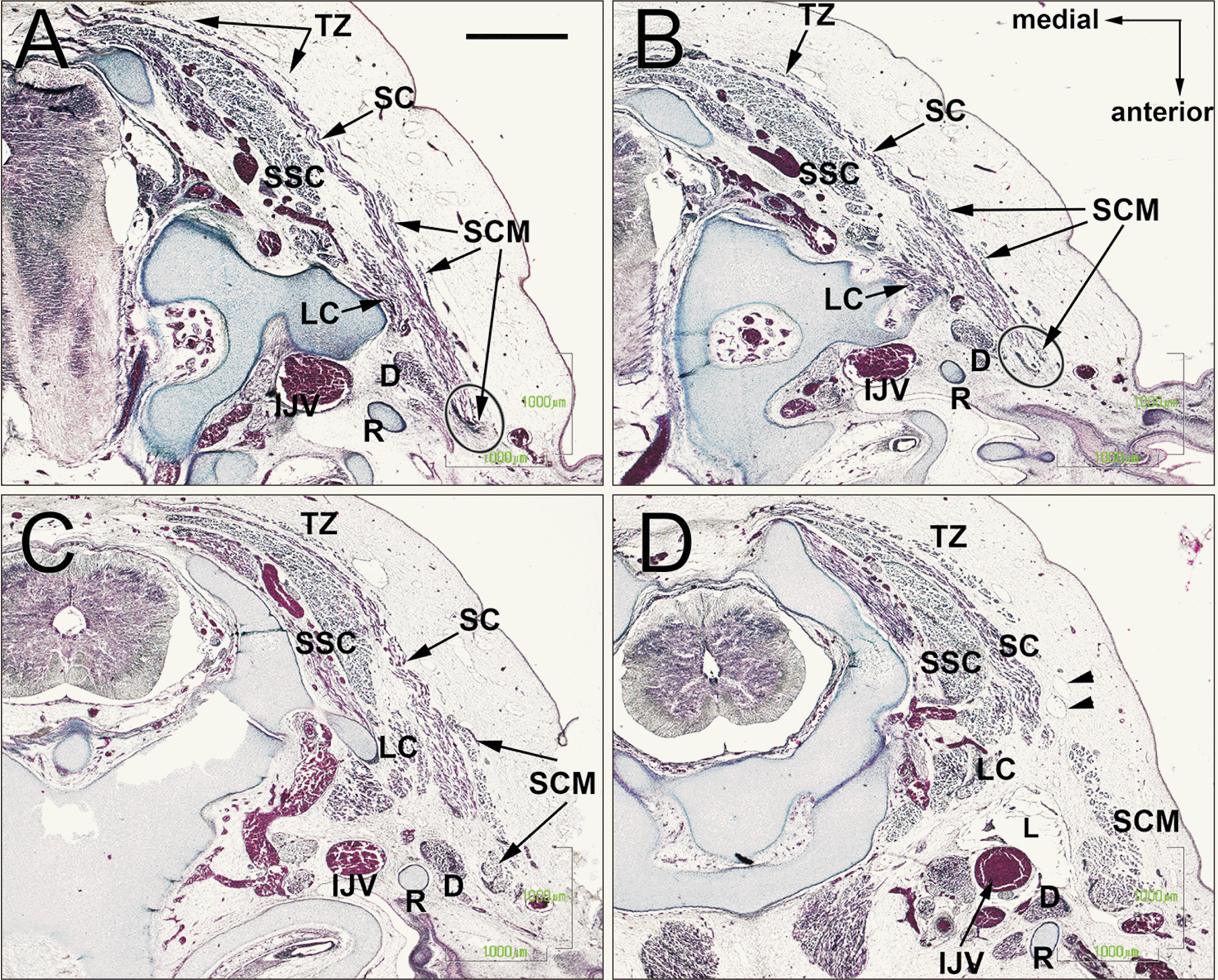 | Fig. 5The upper posterior end of the SCM connecting the splenius capitis muscle. CS 23 (8 WD) human embryo (maximum length, 27 mm). Horizontal sections. (A or D) is the most cranial (caudal) side of the figure. Depending on the upper growth of the digastricus muscle, the splenius muscle was sandwiched between the digastricus and SCM muscles (A, B). The upper posterior end of the SCM was attached to the splenius muscle (A, B, oval). Cutaneous lymphatic vessels had entered the deep tissue between the SCM and trapezius muscles (D, arrow heads). CS, Carnegie stage; D, digastric muscle; IJV, internal jugular vein; LC, longissimus capitis; R, Reichert’s cartilage (second pharyngeal cartilage); SC, splenius capitis muscle; SCM, sternocleidomastoid muscle; SSC, semispinalis cervicis; TZ, trapezius muscle; WD, weeks of development. |
Discussion
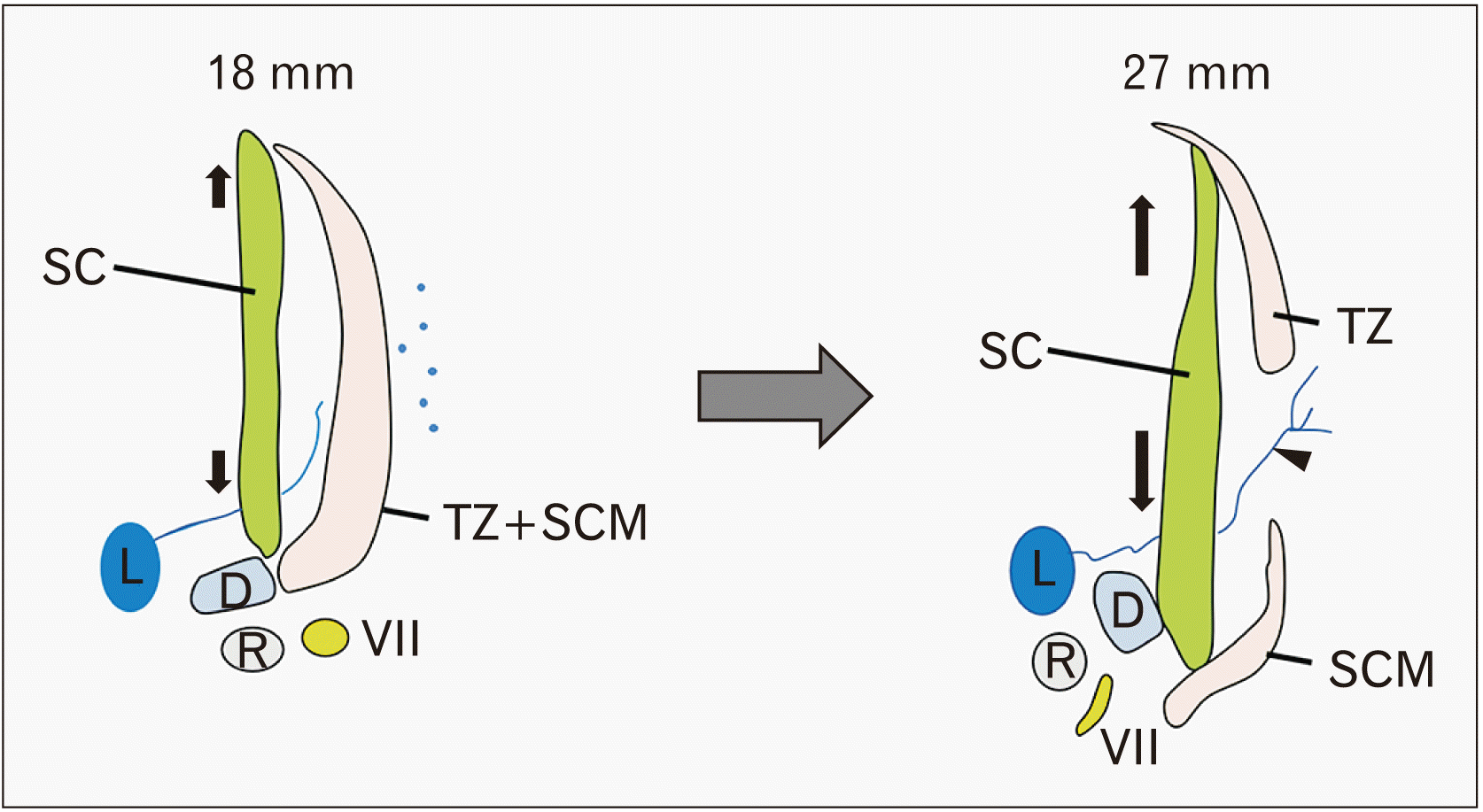 | Fig. 6Schematic representations of the muscle splitting. Growing of the splenius capitis muscle might allow a single mesenchymal condensation of the SCM and trapezius muscles to divide. D, digastric muscle; L, lymphatic vessels; R, Reichert’s cartilage (second pharyngeal cartilage); SC, splenius capitis muscle; SCM, sternocleidomastoid muscle; TZ, trapezius muscle; VII, facial nerve. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download