This article has been
cited by other articles in ScienceCentral.
Abstract
Sciatic nerve (SN) variaitons can result in interesting clinical presentations. We identified a SN variant that does not fit into preexisting classification schemes. In an adult male cadaver, the SN was found to divide proximally and partly exit through the piriformis muscle. Distal to the piriformis, the two parts of the SN were reunited. Although apparently extremely rare, such a finding should be added to the archives of anatomical variations.
Go to :

Keywords: Anatomy, Anatomic variation, Cadaver, Sciatic nerve
Introduction
The sciatic nerve (SN) is the largest nerve in the human body (approximately 2 centimeters in diameter) [
1]. It arises from the ventral rami of L4-S3, is comprised of both motor and sensory components, and supplies the majority of the lower limb via its tibial nerve (TN) and common fibular nerve (CFN) components.
In their study of 120 cadavers, Beaton and Anson [
2] were the first to characterize the different anatomical variants of SN bifurcation in relation to the piriformis muscle (PM). They concluded that the vast majority of their cadavers demonstrated, bilaterally, an unbranched SN that exits distally below the piriformis (type A) – this is considered the normal relationship between the nerve and muscle [
3]. The most prevalent SN variant demonstrates branching proximal to the piriformis and exits through the muscle as the CFN while the TN arises inferiorly (type B) [
2]. The second most common variant (type C) demonstrates the CFN and TN arising superiorly and inferiorly to the undivided PM, respectively [
4]. Types D–F showcase other variations that are seen less frequently [
2,
5]. Herein, we report a unique variant of the SN.
Go to :

CASE REPORT
A variant SN was found during the routine dissection of a male Caucasian cadaver aged 72-years-old at death. The right SN was split by the PM into two roots, a superior CFN part and an inferior TN part (
Fig. 1). The superior root was found to pierce the piriformis and the inferior root traveled inferior to the inferior border of the piriformis (infrapiriformis foramen). Distal (approximately 3 cm) to the piriformis, the CFN and TN were united by a communicating branch (Cb) descending superomedial to inferolateral in an oblique manner. The Cb was approximately 1 cm in width by 3 cm in length. Distal to this Cb, the CFN and TN continued toward the popliteal fossa and were distributed in normal fashion to the anterior/lateral and posterior compartments of the leg, respectively. No atrophy or other pathology was noted in any muscles of the leg and no other anatomical variations of the left or right lower limbs (including gluteal region) were noted.
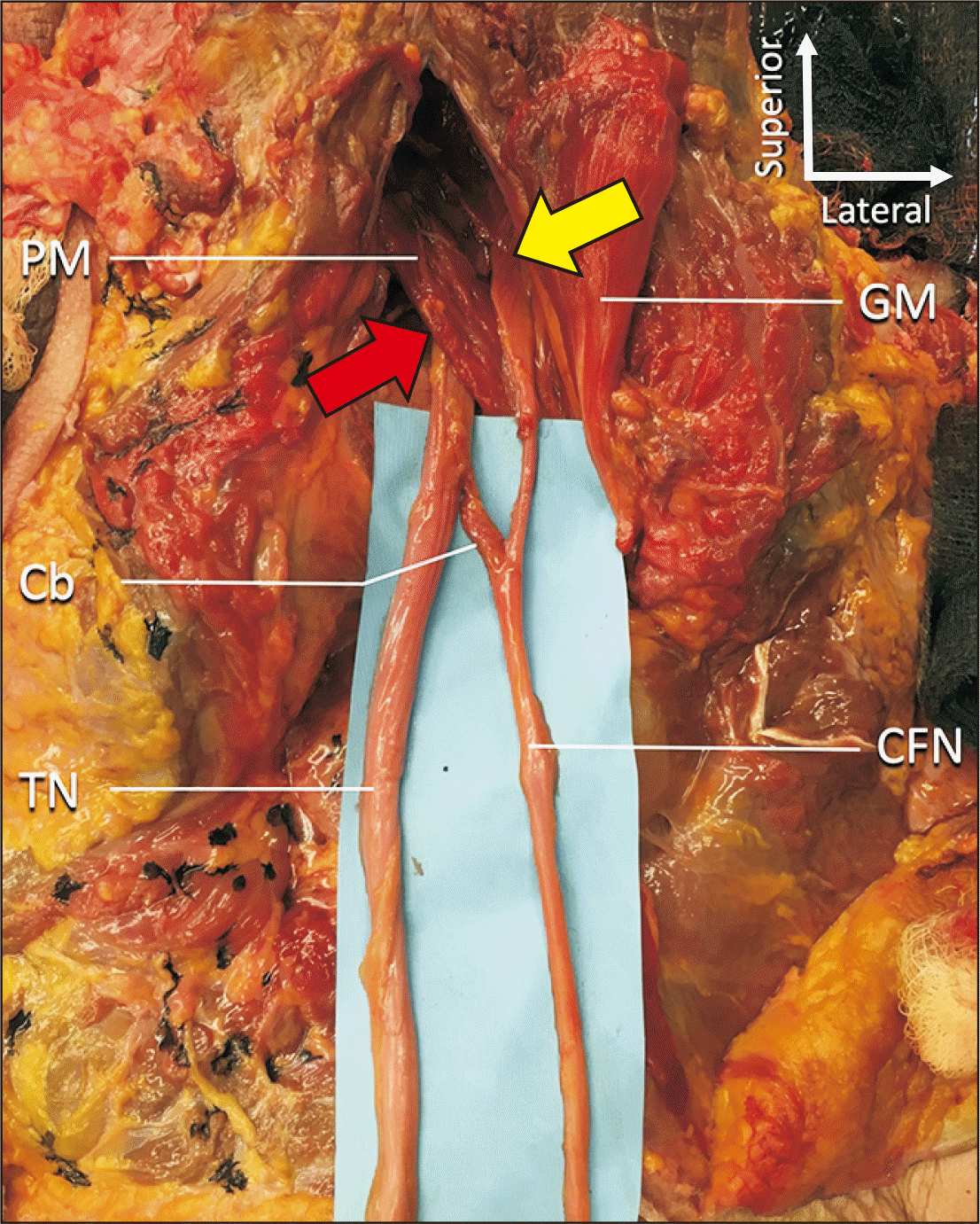 | Fig. 1Gluteal and posterior thigh regions on the right side. The GM is reflected laterally. The superior part (CFN) of the sciatic nerve is seen piercing the PM (yellow arrow) and the inferior root (TN) runs below the inferior border of the PM (red arrow). Note the Cb between the two. Cb, communicating branch; CFN, common fibular nerve; GM, gluteus maximus; PM, piriformis muscle; TN, tibial nerve. 
|
Go to :

Discussion
The manner in which the lumbosacral plexus creates the SN and how the SN branches into the tibial and CFNs represent variations that can have a significant clinical and therapeutic impact. A study by Antoniadis reported [
6] that 25% of symptomatic SN lesions were iatrogenic, whether through surgical trauma or anesthetic mishaps, highlighting the difficulty in identifying and isolating the SN. Just as Dupont et al. [
7] suggest, a thorough survey of a patient’s SN course needs to be appreciated prior to treatment in order to minimize the risk of iatrogenic injury, which must be preceeded by an understaning of all the possible SN variations.
Beyond the Beaton and Anson [
2] classification, others have noted different variaitons of the SN at the piriformis. For example, the passage of the CFN through the infrapiriform foramen, and the TN under the superior gemellus i.e., a seventh variant [
5]. To our knowledge, our cadaver represents a possible eighth variation and the first example where the SN is split at the PM to then reunite distal to the muscle.
In addition to its relation with the PM, SN variations can also be grouped based on other factors such as its level of bifurcation and its relation with other structures such as blood supply [
1]. The SN may even undergo ‘trifurcations’ where the additional segment becomes a sural nerve or a branch of the perineal nerve [
8]. Other times, the ventral rami of L4-S3 may not coalesce into one large SN until deep in the lower part of the gluteal region – a variation that can have broad implications in surgery and anesthesiology when conducting nerve blockade [
9]. SN divisions proximal to the PM, so called ‘high divisions’, are not uncommon and can be present on one or both sides [
1]. A notable finding is that anatomical variations of the SN have not been associated with sex [
10].
The SN is involved in conditions such as nondiscogenic sciatica and piriformis syndrome. Symptoms of sciatica include sharp lower back pain that radiates down the posterior leg [
11], and piriformis syndrome, characterized by SN entrapment or compression by the PM [
12], resulting in pain and tingling from the buttocks to the legs. The different anatomical variations of the SN can lead to challenges in terms of therapeutic and diagnostic care of these conditions. For example, while there exists numerous locations where the SN can be blocked, some are safer than others. Variations in the anatomy of the SN can lead to higher rates of incomplete blockade, which can result in continued pain, nerve injury, blood vessel traumatization, and local anesthetic toxicity [
13]. For example, Ro and Edmonds presented a case in which a patient with the type B SN arrangement failed to respond to conservative treatment, making surgery the best option [
14]. Their case supports the argument that the different variations may require individualized treatment approaches.
In conclusion, an understanding of the different variants of the SN in regard to the level of division around the PM is of clinical significance. Its functionality and pathology spans several disciplines in medicine such as neurosurgery, neurology, and orthopedics. This case illustration highlights an apparently rare variant of the SN.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download