Introduction
Materials and Methods
Extraction of essential oil of Cymbopogon citratus
Animal grouping and treatments
Open field test
Tissue processing for histological and histochemical analyses
Biochemical assays for enzymatic analyses
Photomicrography and statistical analysis
Results
ESO reversed AlCl3-induced dysregulation of general motor functions and exploratory drive in rats
 | Fig. 1(A–E) Effects of ESO administration on exploratory drive and locomotor activities in treated animals. (A) Analysis of the number of lines crossed by rats in the open field test showing no significant difference between the numbers of lines crossed by PBS and ESO controls. AlCl3 treated rats crossed the least number of lines relative to other groups. AlCl3+ESO and ESO+AlCl3 groups significantly crossed more lines than the AlCl3 treated rats (both at P<0.05). (B) Analysis of central square entry frequency in the open field test showed that rats treated AlCl3 made the least entry to the central square of the open field compared to all of PBS (P<0.05), ESO (P<0.05), AlCl3+ESO (P<0.05), and ESO+AlCl3 (P<0.05) groups. (C) Analysis of central square duration revealed that the administration of AlCl3 reduced the time rats spent in the central square when compared to the PBS (P<0.05) and ESO (P<0.05) controls. However, ESO significantly improved the central square duration of rats in the ESO+AlCl3 treated rats (P<0.05) compared to the AlCl3 group. (D) Rearing frequency analysis of experimental animals showing significant reductions in both groups treated AlCl3 compared to other groups. ESO intervention improved rearing frequencies in rats in the AlCl3+ESO and ESO+AlCl3 groups (at P<0.05, respectively) compared to the AlCl3 group. (E) Analysis of the stretch-attend posture frequency shows significant elevation in rats treated AlCl3 treated compared to PBS and ESO control (both at P<0.05). However, the AlCl3+ESO and ESO+AlCl3 treated rats showed improved stretch-attend-posture, which is significantly different (both at P<0.05) from the AlCl3 group. The results are presented as mean±standard error of mean. AlCl3, aluminum chloride; ESO, essential oil; PBS, phosphate-buffered saline. *P<0.05. |
Effects of ESO on AlCl3-induced perturbation of cerebellar redox
 | Fig. 2Cerebellar oxidative redox state characterized by (A) SOD, (B) CAT, (C) GPx, (D) MDA, and (E) NO activities respectively. (A) Cerebellar SOD activity was significantly depleted by AlCl3 treatment relative to both PBS (P<0.05) and ESO groups (P<0.05) but was normalized by ESO treatment in ESO+AlCl3 (P<0.05) and AlCl3 (P<0.05) groups. (B) CAT level was increased by AlCl3 relative to both PBS and ESO controls (P<0.05) but regularized by ESO treatment in both AlCl3+ESO and ESO+AlCl3 groups at P<0.05. (C) AlCl3 treatment induced an increase in GPx level relative to the PBS and ESO groups at P<0.05 for both. ESO treatment restored the level of GPx in AlCl3+ESO (P<0.05) to normal levels but not ESO+AlCl3 group. (D) AlCl3 treatment triggered a significant increase in the level of MDA when compared to the PBS (P<0.05) and ESO (P<0.05) controls. This was normalized in the AlCl3+ESO (P<0.05) and ESO+AlCl3 (P<0.05) groups. (C) AlCl3 treatment elicited a striking increase in NO activities relative to the PBS and ESO groups (both at P<0.05) and was regularized by ESO in both AlCl3+ESO and ESO+AlCl3 groups (at P<0.05 each). AlCl3, aluminum chloride; CAT, catalase; ESO, essential oil; GPx, glutathione peroxidase; MDA, malondialdehyde; NO, nitric oxide; PBS, phosphate-buffered saline; SOD, superoxide dismutase. *P<0.05. |
Cerebellar G6PDH, LDH, and glucokinase expressions following AlCl3 and ESO treatments in rats
 | Fig. 3Cerebellar G6PDH (A), LDH (B), GSA (C), TRP (D), and AChE (E) activities under treatment conditions. (A) AlCl3 treatment caused depletion in G6PDH expression relative to the PBS and ESO groups (P>0.05 in both). Pre- and post-treatment with ESO normalized G6PDH levels in the AlCl3+ESO (P<0.05) and ESO+AlCl3 (P<0.05) groups. (B) Expectantly, AlCl3 treatment induced a significant decrease in the level of LDH compared to the PBS, and ESO controls both at P<0.05. ESO treatment prevented LDH dysregulation in AlCl3+ESO (P<0.05) and ESO+AlCl3 (P<0.05) treated rats. (C) Cerebral GSA activity was significantly depleted in AlCl3 treated rats when compared to PBS (P<0.05), ESO (P<0.05), AlCl3+ESO (P<0.05) and ESO+AlCl3 (P<0.05) treated rats. (D) The expressed level of TRP in AlCl3 treated rats was significantly higher than in PBS, and ESO controls at P<0.05 each, as well as in AlCl3+ESO and ESO+AlCl3 treated rats (both at P<0.05). When compared to all other treated groups, AlCl3 treated rats presented with the highest level of AChE, with significant modulation seen following ESO intervention in the AlCl3+ESO and ESO+AlCl3 both at P<0.05. AChE, acetylcholinesterase; AlCl3, aluminum chloride; ESO, essential oil; GSA, glucokinase specific activity; G6PDH, glucose-6-phosphate dehydrogenase; LDH, lactate dehydrogenase; PBS, phosphate-buffered saline; TRP, transferrin receptor protein.*P<0.05. |
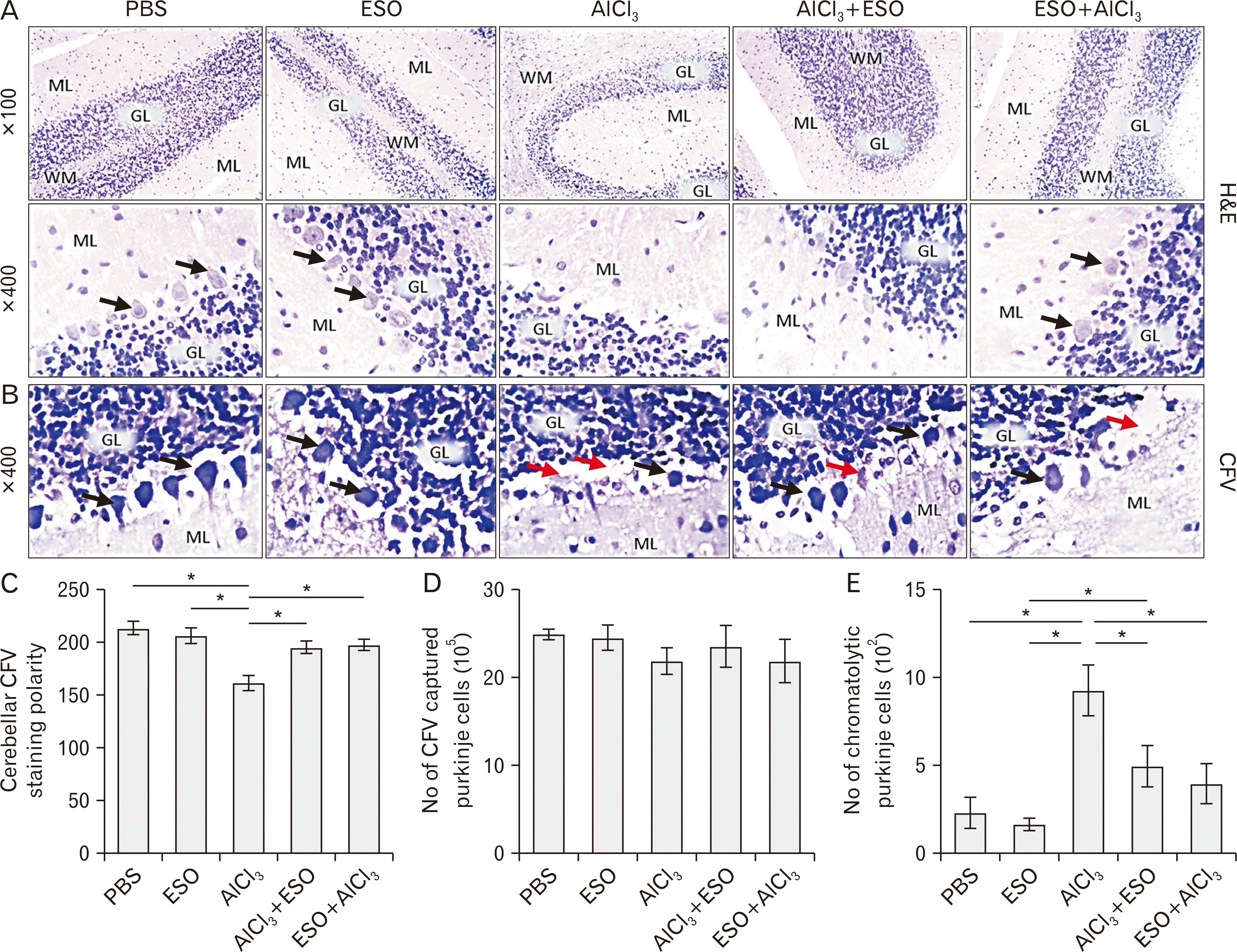 | Fig. 4(A–E) Representative photomicrographs showing panoramic views of cerebellar cortex general morphological presentations and Nissl profile in Wistar rats across the various study groups. (A) is H&E (×100, ×400); The ML, Purkinje cells (black arrowhead) layer, GL, and the medullary layer of WM are demonstrated across study groups. PBS and ESO treatments did not alter the morphological presentation of cerebellar layers from this study. Both groups show a fine array of cells that are distinctly arranged from the molecular to the granular layer. Also, cellular density within the groups appears normal across all cortical layers. AlCl3 treatment caused degenerative changes characterized by fragmented GL in cerebellar cortex and poorly stained Purkinje cells. Comparatively, increased cellular density was observed in this group. Groups administered ESO before and after AlCl3-induced toxicity (AlCl3+ESO and ESO+AlCl3) shows mild degenerative changes in line of morphologic appearance of cerebellar sections of AlCl3-treated rats; cerebellar layers in both groups looks better structured and layers are well-delineated. (B) shows Nissl profile as demonstrated by CFV stain (×400). Normal morphological presentations of the cerebellar cortex are observable in PBS and ESO treated groups that are characterized by well-defined staining profiles. Cellular morphology in these groups is characterized by Purkinje cells with conspicuous cell bodies and dendrites that are projecting deep into the MLs (black arrowhead). Also, the granule layer in these groups consists of small granule neurons which are well outlined in contrast to the loosely arranged and cryptic cells in the granule layers of groups AlCl3 treated groups. Degenerating Purkinje cells with chromatolytic cell bodies (red arrowhead) and short dendritic processes can be seen around the indistinctly demarcated cerebellar layers of groups that receive aluminum treatment. The transitional regions between the cerebellar layers of AlCl3 treated with ESO are also generally better delineated. Although a few perineural spaces are present around Purkinje neurons of this groups, the general cytoarchitecture is much similar to that of the PBS and ESO groups. (C–E) show quantitative analysis of the CFV stain. AlCl3, aluminum chloride; CFV, Cresyl Fast Violet; ESO, essential oil; GL, granule cell layer; ML, molecular layer; PBS, phosphate-buffered saline; WM, white matter.*P<0.05. |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download