1. Khazaei M, Salehi H. 2006; Protective effect of falcaria vulgaris extract on ethanol induced gastric ulcer in rat. Iran J Pharmacol Ther. 5:43–6.
2. Bayir Y, Odabasoglu F, Cakir A, Aslan A, Suleyman H, Halici M, Kazaz C. 2006; The inhibition of gastric mucosal lesion, oxidative stress and neutrophil-infiltration in rats by the lichen constituent diffractaic acid. Phytomedicine. 13:584–90. DOI:
10.1016/j.phymed.2005.07.002. PMID:
16920514.

3. Bonamin F, Moraes TM, Dos Santos RC, Kushima H, Faria FM, Silva MA, Junior IV, Nogueira L, Bauab TM, Souza Brito AR, da Rocha LR, Hiruma-Lima CA. 2014; The effect of a minor constituent of essential oil from Citrus aurantium: the role of β-myrcene in preventing peptic ulcer disease. Chem Biol Interact. 212:11–9. DOI:
10.1016/j.cbi.2014.01.009. PMID:
24480520.

4. Lima ZP, Severi JA, Pellizzon CH, Brito AR, Solis PN, Cáceres A, Girón LM, Vilegas W, Hiruma-Lima CA. 2006; Can the aqueous decoction of mango flowers be used as an antiulcer agent? J Ethnopharmacol. 106:29–37. DOI:
10.1016/j.jep.2005.11.032. PMID:
16500058.

5. Valle JD. Fauci AS, Harrison TR, editors. 2008. Peptic ulcer disease and related disorders. Harrison's principles of internal medicine. 17th ed. McGraw-Hill;New York: p. 1855–1872.
8. Wallace JL, Miller MJ. 2000; Nitric oxide in mucosal defense: a little goes a long way. Gastroenterology. 119:512–20. DOI:
10.1053/gast.2000.9304. PMID:
10930387.

9. Ohta Y, Nishida K. 2003; Protective effect of coadministered superoxide dismutase and catalase against stress-induced gastric mucosal lesions. Clin Exp Pharmacol Physiol. 30:545–50. DOI:
10.1046/j.1440-1681.2003.03871.x. PMID:
12890175.
10. Aragón JP, Condit ME, Bhushan S, Predmore BL, Patel SS, Grinsfelder DB, Gundewar S, Jha S, Calvert JW, Barouch LA, Lavu M, Wright HM, Lefer DJ. 2011; Beta3-adrenoreceptor stimulation ameliorates myocardial ischemia-reperfusion injury via endothelial nitric oxide synthase and neuronal nitric oxide synthase activation. J Am Coll Cardiol. 58:2683–91. DOI:
10.1016/j.jacc.2011.09.033. PMID:
22152956. PMCID:
PMC3586978.

11. Sorrentino SA, Doerries C, Manes C, Speer T, Dessy C, Lobysheva I, Mohmand W, Akbar R, Bahlmann F, Besler C, Schaefer A, Hilfiker-Kleiner D, Lüscher TF, Balligand JL, Drexler H, Landmesser U. 2011; Nebivolol exerts beneficial effects on endothelial function, early endothelial progenitor cells, myocardial neovascularization, and left ventricular dysfunction early after myocardial infarction beyond conventional β1-blockade. J Am Coll Cardiol. 57:601–11. DOI:
10.1016/j.jacc.2010.09.037. PMID:
21272752.

13. Corsini A, Maggi FM, Catapano AL. 1995; Pharmacology of competitive inhibitors of HMG-CoA reductase. Pharmacol Res. 31:9–27. DOI:
10.1016/1043-6618(95)80042-5.

14. Liao JK. 2005; Effects of statins on 3-hydroxy-3-methylglutaryl coenzyme a reductase inhibition beyond low-density lipoprotein cholesterol. Am J Cardiol. 96(5A):24F–33F. DOI:
10.1016/j.amjcard.2005.06.009. PMID:
16126020. PMCID:
PMC2684977.

15. Trochu JN, Mital S, Zhang Xp, Xu X, Ochoa M, Liao JK, Recchia FA, Hintze TH. 2003; Preservation of NO production by statins in the treatment of heart failure. Cardiovasc Res. 60:250–8. DOI:
10.1016/j.cardiores.2003.08.003. PMID:
14613854. PMCID:
PMC2653218.

16. Schrör K, Löbel P, Steinhagen-Thiessen E. 1989; Simvastatin reduces platelet thromboxane formation and restores normal platelet sensitivity against prostacyclin in type IIa hypercholesterolemia. Eicosanoids. 2:39–45. PMID:
2483819.
17. Ungureanu D, Filip C, Artenie A, Artenie R. 2003; Evaluation of simvastatin antioxidant effects. Rev Med Chir Soc Med Nat Iasi. 107:66–71.
18. Institute for Laboratory Animal Research. National Academies Press. 2011. Guide for the care and use of laboratory animals. 8th ed. National Academies Press;Washington: p. 220.
19. Dronjak S, Gavrilović L, Filipović D, Radojcić MB. 2004; Immobilization and cold stress affect sympatho-adrenomedullary system and pituitary-adrenocortical axis of rats exposed to long-term isolation and crowding. Physiol Behav. 81:409–15. DOI:
10.1016/j.physbeh.2004.01.011. PMID:
15135012.

20. Das D, Banerjee RK. 1993; Effect of stress on the antioxidant enzymes and gastric ulceration. Mol Cell Biochem. 125:115–25. DOI:
10.1007/BF00936440. PMID:
8283967.

21. Bahgat AK. 2009; Gastroprotective effect of L-carnitine on indomethacin-induced gastric ulcer in rats: the involvement of antioxidant mechanisms and nitric oxide. Med J Cairo Univ. 77:43–51.
22. Abd El Motteleb DM, Hasan MM. 2011; Gastroprotective effect of simvastatin against experimentally induced gastric ulcers in rats: role of ATP-sensitive K+ channels. J Am Sci. 7:760–8.
23. Morsy MA, Heeba GH, Abdelwahab SA, Rofaeil RR. 2012; Protective effects of nebivolol against cold restraint stress-induced gastric ulcer in rats: role of NO, HO-1, and COX-1,2. Nitric Oxide. 27:117–22. DOI:
10.1016/j.niox.2012.06.001. PMID:
22687651.

25. Ohkawa H, Ohishi N, Yagi K. 1979; Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 95:351–8. DOI:
10.1016/0003-2697(79)90738-3. PMID:
36810.

27. Habig WH, Pabst MJ, Jakoby WB. 1974; Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 249:7130–9. PMID:
4436300.
28. Hamberg M, Samuelsson B. 1973; Detection and isolation of an endoperoxide intermediate in prostaglandin biosynthesis. Proc Natl Acad Sci U S A. 70:899–903. DOI:
10.1073/pnas.70.3.899. PMID:
4514999. PMCID:
PMC433384.

29. Miranda KM, Espey MG, Wink DA. 2001; A rapid, simple spectrophotometric method for simultaneous detection of nitrate and nitrite. Nitric Oxide. 5:62–71. DOI:
10.1006/niox.2000.0319. PMID:
11178938.

30. Zhang H, Li X, Ding J, Xu H, Dai X, Hou Z, Zhang K, Sun K, Sun W. 2013; Delivery of ursolic acid (UA) in polymeric nanoparticles effectively promotes the apoptosis of gastric cancer cells through enhanced inhibition of cyclooxygenase 2 (COX-2). Int J Pharm. 441:261–8. DOI:
10.1016/j.ijpharm.2012.11.034. PMID:
23194884.

31. Altman GD. Altman GD, editor. 2005. Three or more independent groups of observations. Practical statistics for medical research. 2nd ed. Chapman & Hall;London: p. 205–17.
32. Kwiecień S, Brzozowski T, Konturek SJ. 2002; Effects of reactive oxygen species action on gastric mucosa in various models of mucosal injury. J Physiol Pharmacol. 53:39–50. DOI:
10.1139/apnm-2017-0138. PMID:
11939718.
33. Brzozowski T, Konturek PC, Pajdo R, Ptak-Belowska A, Kwiecien S, Pawlik M, Drozdowicz D, Sliwowski Z, Brzozowski B, Konturek SJ, Pawlik WW. 2008; Physiological mediators in nonsteroidal anti-inflammatory drugs (NSAIDs)-induced impairment of gastric mucosal defense and adaptation. Focus on nitric oxide and lipoxins. J Physiol Pharmacol. 59 Suppl 2:89–102. PMID:
18812631.
34. Haendeler J, Hoffmann J, Zeiher AM, Dimmeler S. 2004; Antioxidant effects of statins via S-nitrosylation and activation of thioredoxin in endothelial cells: a novel vasculoprotective function of statins. Circulation. 110:856–61. DOI:
10.1161/01.CIR.0000138743.09012.93. PMID:
15289372.
35. Tariq M, Khan HA, Elfaki I, Arshaduddin M, Al Moutaery M, Al Rayes H, Al Swailam R. 2007; Gastric antisecretory and antiulcer effects of simvastatin in rats. J Gastroenterol Hepatol. 22:2316–23. DOI:
10.1111/j.1440-1746.2007.05021.x. PMID:
17593225.

36. Matsui H, Shimokawa O, Kaneko T, Nagano Y, Rai K, Hyodo I. 2011; The pathophysiology of non-steroidal anti-inflammatory drug (NSAID)-induced mucosal injuries in stomach and small intestine. J Clin Biochem Nutr. 48:107–11. DOI:
10.3164/jcbn.10-79. PMID:
21373261. PMCID:
PMC3045681.

37. Bjarnason I, Scarpignato C, Takeuchi K, Rainsford KD. 2007; Determinants of the short-term gastric damage caused by NSAIDs in man. Aliment Pharmacol Ther. 26:95–106. DOI:
10.1111/j.1365-2036.2007.03348.x. PMID:
17555426.

39. Kato S, Ohkawa F, Ito Y, Amagase K, Takeuchi K. 2009; Role of endothelial nitric oxide synthase in aggravation of indomethacin-induced gastric damage in adjuvant arthritic rats. J Physiol Pharmacol. 60:147–55. PMID:
20065509.
41. Lanas A. 2008; Role of nitric oxide in the gastrointestinal tract. Arthritis Res Ther. 10(Suppl 2):S4. DOI:
10.1186/ar2465. PMID:
19007429.

42. Goel R, Goel A, Manocha A, Pillai KK, Srivastava RS. 2009; Influence of nebivolol on anticonvulsant effect of lamotrigine. Indian J Pharmacol. 41:41–6. DOI:
10.4103/0253-7613.48890. PMID:
20177581. PMCID:
PMC2825014.

43. Ceron CS, Rizzi E, Guimarães DA, Martins-Oliveira A, Gerlach RF, Tanus-Santos JE. 2013; Nebivolol attenuates prooxidant and profibrotic mechanisms involving TGF-β and MMPs, and decreases vascular remodeling in renovascular hypertension. Free Radic Biol Med. 65:47–56. DOI:
10.1016/j.freeradbiomed.2013.06.033. PMID:
23806385.

44. Rizzi E, Guimaraes DA, Ceron CS, Prado CM, Pinheiro LC, Martins-Oliveira A, Gerlach RF, Tanus-Santos JE. 2014; β1-Adrenergic blockers exert antioxidant effects, reduce matrix metalloproteinase activity, and improve renovascular hypertension-induced cardiac hypertrophy. Free Radic Biol Med. 73:308–17. DOI:
10.1016/j.freeradbiomed.2014.05.024. PMID:
24933619.

45. Dursun S, Çuhadar S, Köseoğlu M, Atay A, Aktaş SG. 2014; The anti-inflammatory and antioxidant effects of pravastatin and nebivolol in rat aorta. Anadolu Kardiyol Derg. 14:229–33. DOI:
10.5152/akd.2013.4708. PMID:
24936540.

46. Uzar E, Acar A, Evliyaoğlu O, Fırat U, Kamasak K, Göçmez C, Alp H, Tüfek A, Taşdemir N, Ilhan A. 2012; The anti-oxidant and anti-apoptotic effects of nebivolol and zofenopril in a model of cerebral ischemia/reperfusion in rats. Prog Neuropsychopharmacol Biol Psychiatry. 36:22–8. DOI:
10.1016/j.pnpbp.2011.08.011. PMID:
21888941.

47. Whaley-Connell A, Habibi J, Johnson M, Tilmon R, Rehmer N, Rehmer J, Wiedmeyer C, Ferrario CM, Sowers JR. 2009; Nebivolol reduces proteinuria and renal NADPH oxidase-generated reactive oxygen species in the transgenic Ren2 rat. Am J Nephrol. 30:354–60. DOI:
10.1159/000229305. PMID:
19609077. PMCID:
PMC2814025.

48. Abd Allah OM, Sharaf El-Din AAI. 2016; Nebivolol ameliorates indomethacin-induced gastric ulcer in adult albino rats: role of inducible nitric oxide synthase. Egypt J Forensic Sci Appli Toxicol. 16:147–67. DOI:
10.21608/ejfsat.2016.41023.

49. Parenti A, Filippi S, Amerini S, Granger HJ, Fazzini A, Ledda F. 2000; Inositol phosphate metabolism and nitric-oxide synthase activity in endothelial cells are involved in the vasorelaxant activity of nebivolol. J Pharmacol Exp Ther. 292:698–703. PMID:
10640308.
50. Kalinowski L, Dobrucki LW, Szczepanska-Konkel M, Jankowski M, Martyniec L, Angielski S, Malinski T. 2003; Third-generation beta-blockers stimulate nitric oxide release from endothelial cells through ATP efflux: a novel mechanism for antihypertensive action. Circulation. 107:2747–52. DOI:
10.1161/01.CIR.0000066912.58385.DE. PMID:
12742996.
51. Zhou X, Ma L, Habibi J, Whaley-Connell A, Hayden MR, Tilmon RD, Brown AN, Kim JA, Demarco VG, Sowers JR. 2010; Nebivolol improves diastolic dysfunction and myocardial remodeling through reductions in oxidative stress in the Zucker obese rat. Hypertension. 55:880–8. DOI:
10.1161/HYPERTENSIONAHA.109.145136. PMID:
20176997. PMCID:
PMC2841702.

52. Manrique C, Lastra G, Habibi J, Pulakat L, Schneider R, Durante W, Tilmon R, Rehmer J, Hayden MR, Ferrario CM, Whaley-Connell A, Sowers JR. 2011; Nebivolol improves insulin sensitivity in the TGR(Ren2)27 rat. Metabolism. 60:1757–66. DOI:
10.1016/j.metabol.2011.04.009. PMID:
21640361. PMCID:
PMC3170670.

53. Martin GR, Wallace JL. 2006; Gastrointestinal inflammation: a central component of mucosal defense and repair. Exp Biol Med (Maywood). 231:130–7. DOI:
10.1177/153537020623100202. PMID:
16446488.

54. Vaseem A, Ali M, Afshan K. 2017; Activity of Tulsi leaves (Ocimum sanctum linn) in protecting gastric ulcer in rats by cold restrain method. Int J Basic Clin Pharmacol. 6:2343–7. DOI:
10.18203/2319-2003.ijbcp20174356.

55. Godara R, Katoch R, Yadav A, Ahanger RR, Bhutyal AD, Verma PK, Katoch M, Dutta S, Nisa F, Singh NK. 2015; In vitro acaricidal activity of ethanolic and aqueous floral extracts of Calendula officinalis against synthetic pyrethroid resistant Rhipicephalus (Boophilus) microplus. Exp Appl Acarol. 67:147–57. DOI:
10.1007/s10493-015-9929-9. PMID:
26071101.

56. Banfi C, Baetta R, Gianazza E, Tremoli E. 2017; Technological advances and proteomic applications in drug discovery and target deconvolution: identification of the pleiotropic effects of statins. Drug Discov Today. 22:848–69. DOI:
10.1016/j.drudis.2017.03.001. PMID:
28284830.

57. Heeba GH, Hassan MK, Amin RS. 2009; Gastroprotective effect of simvastatin against indomethacin-induced gastric ulcer in rats: role of nitric oxide and prostaglandins. Eur J Pharmacol. 607:188–93. DOI:
10.1016/j.ejphar.2009.02.008. PMID:
19217901.

58. Liao WC, Huang MZ, Wang ML, Lin CJ, Lu TL, Lo HR, Pan YJ, Sun YC, Kao MC, Lim HJ, Lai CH. 2017; Statin decreases Helicobacter pylori burden in macrophages by promoting autophagy. Front Cell Infect Microbiol. 6:203. DOI:
10.3389/fcimb.2016.00203. PMID:
28144585.

59. Lin CJ, Liao WC, Chen YA, Lin HJ, Feng CL, Lin CL, Lin YJ, Kao MC, Huang MZ, Lai CH, Kao CH. 2017; Statin therapy is associated with reduced risk of peptic ulcer disease in the Taiwanese population. Front Pharmacol. 8:210. DOI:
10.3389/fphar.2017.00210. PMID:
28503146. PMCID:
PMC5408271.

61. Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. 2009; Aspirin, salicylates, and cancer. Lancet. 373:1301–9. DOI:
10.1016/S0140-6736(09)60243-9.

62. Warzecha Z, Ceranowicz P, Dembinski M, Cieszkowski J, Ginter G, Ptak-Belowska A, Dembinski A. 2014; Involvement of cyclooxygenase-1 and cyclooxygenase-2 activity in the therapeutic effect of ghrelin in the course of ethanol-induced gastric ulcers in rats. J Physiol Pharmacol. 65:95–106. PMID:
24622834.
63. El-Ashmawy NE, Khedr EG, El-Bahrawy HA, Selim HM. 2016; Nebivolol prevents indomethacin-induced gastric ulcer in rats. J Immunotoxicol. 13:580–9. DOI:
10.3109/1547691X.2016.1142488. PMID:
27224860.

64. Rizos E, Bairaktari E, Kostoula A, Hasiotis G, Achimastos A, Ganotakis E, Elisaf M, Mikhailidis DP. 2003; The combination of nebivolol plus pravastatin is associated with a more beneficial metabolic profile compared to that of atenolol plus pravastatin in hypertensive patients with dyslipidemia: a pilot study. J Cardiovasc Pharmacol Ther. 8:127–34. DOI:
10.1177/107424840300800206. PMID:
12808486.

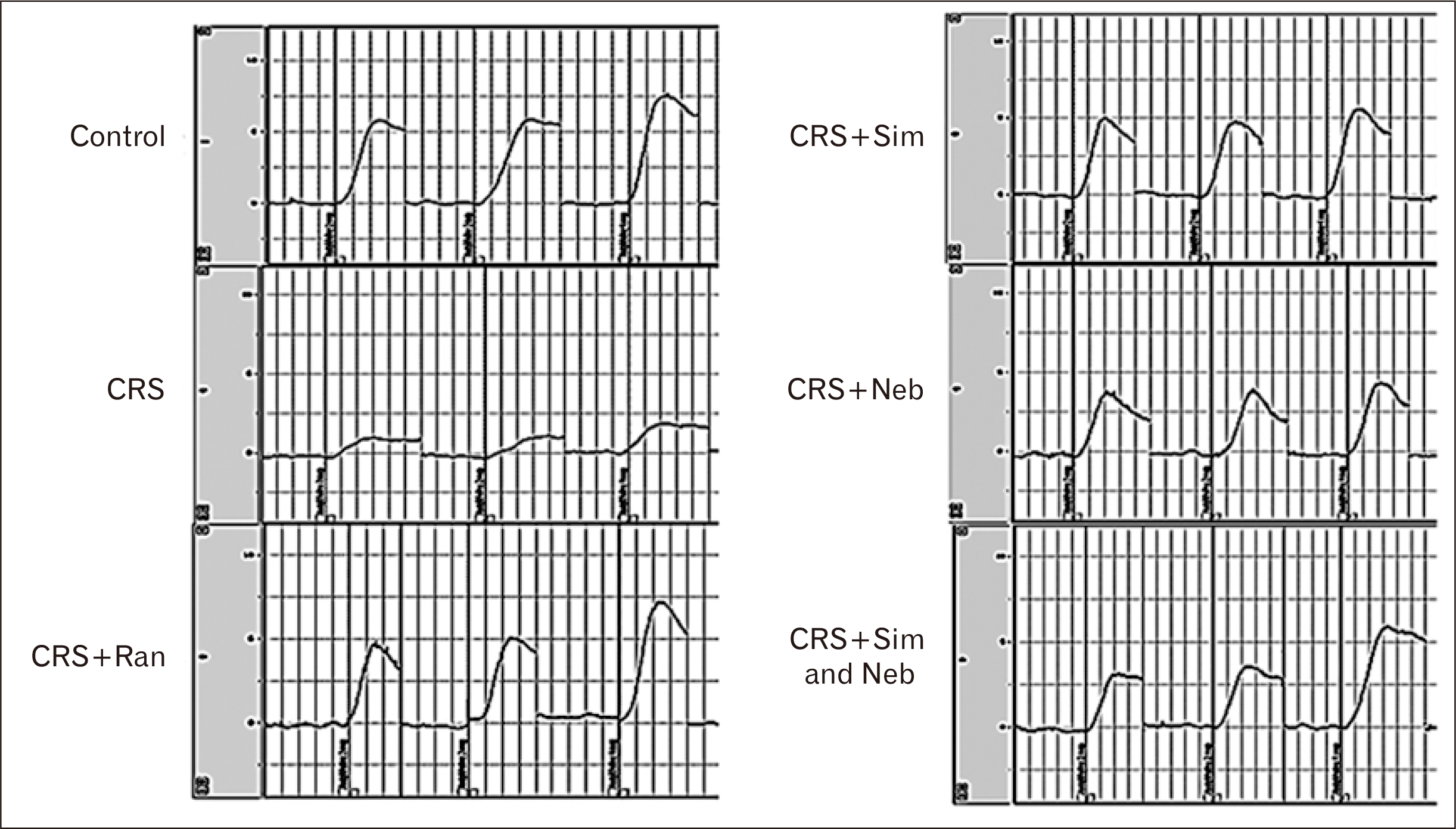
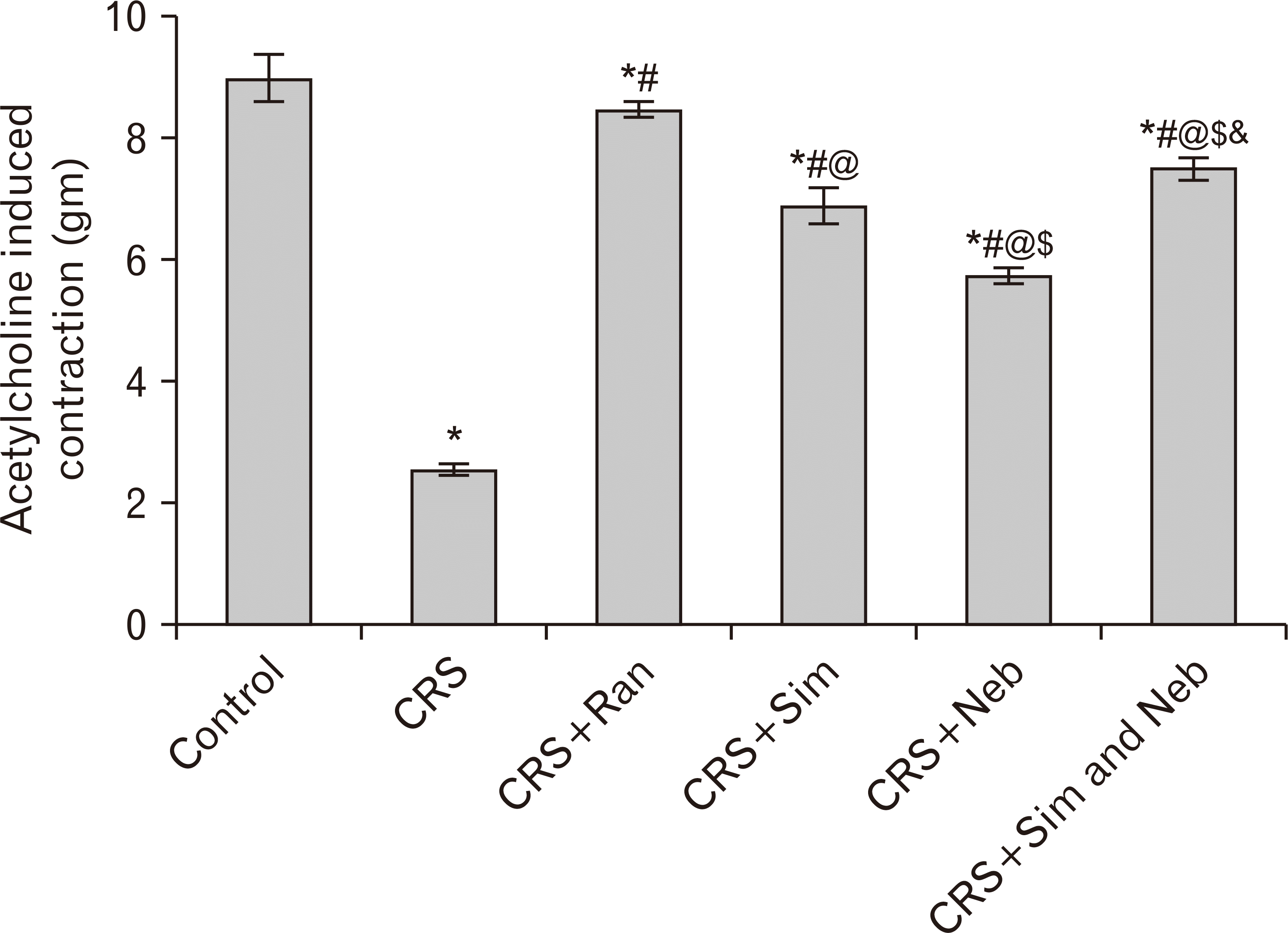
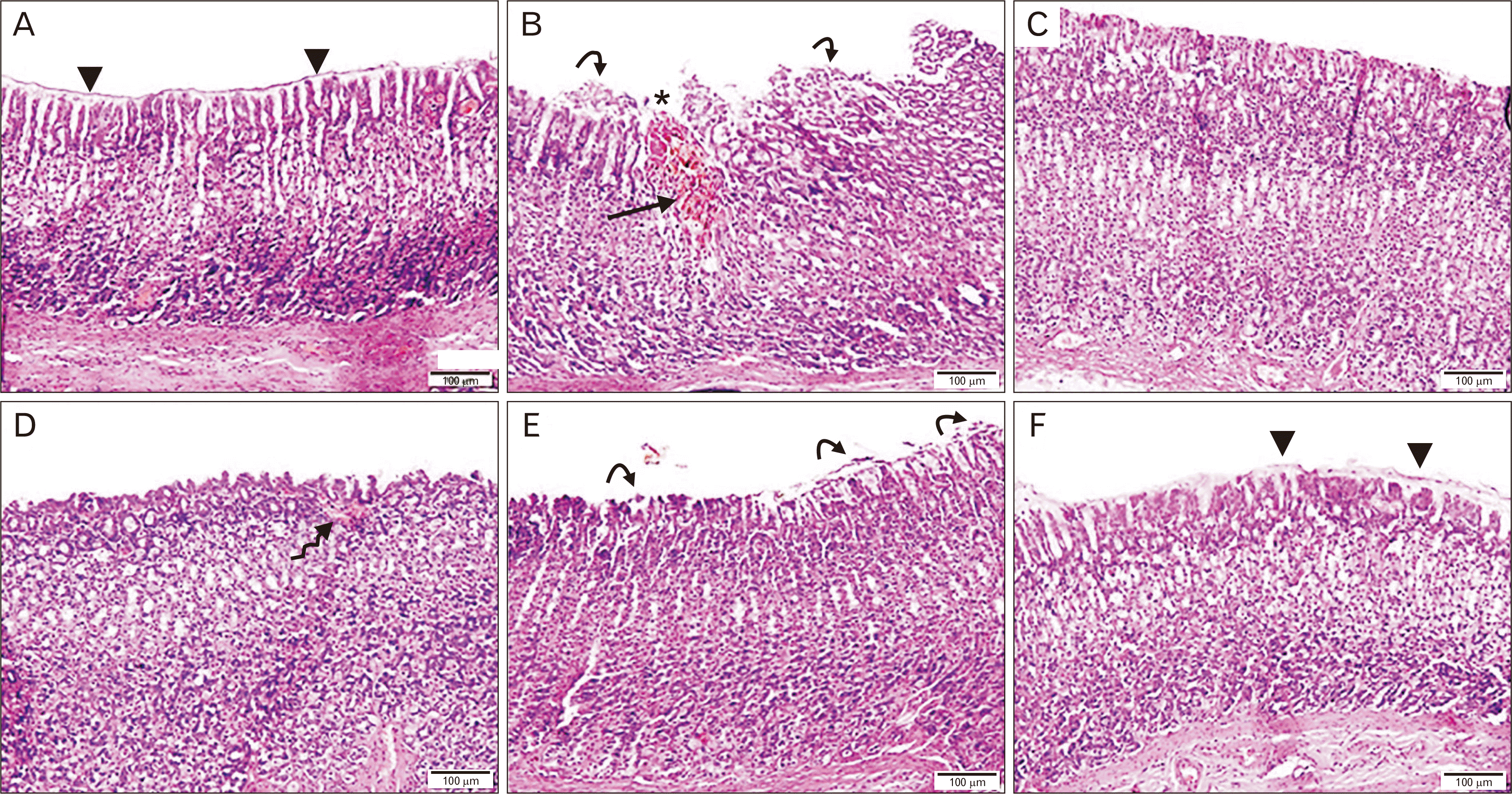
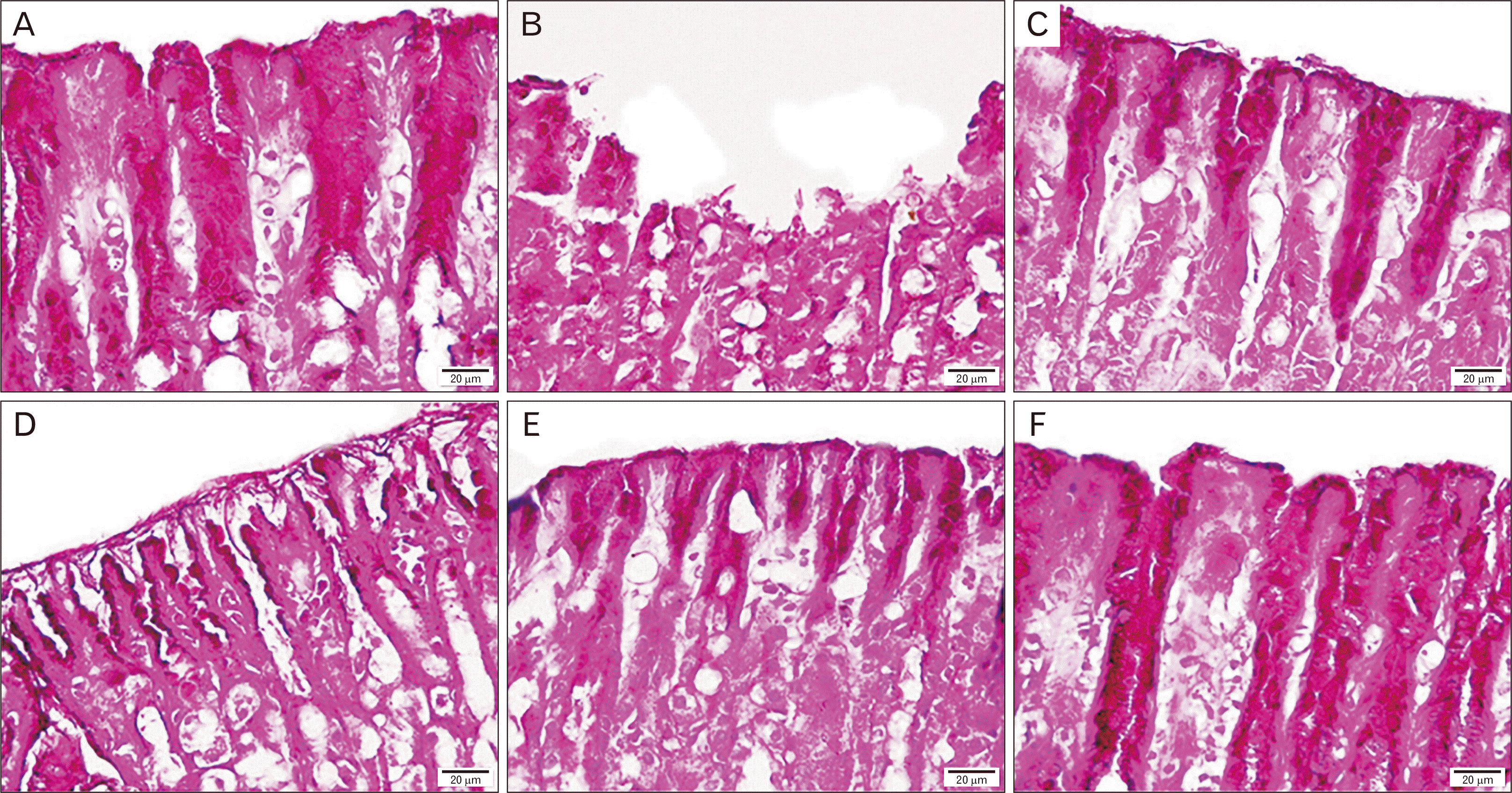




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download