This article has been
cited by other articles in ScienceCentral.
Abstract
Our aim was to investigate the variation in the vertebral levels of the origins of the celiac artery, superior and inferior mesenteric arteries, paired renal arteries, and common iliac arteries. We conducted a retrospective imaging study in a large public secondary hospital on a nonrandom sample of 227 participants. We consecutively included adult patients who had undergone computed tomography angiography of the abdomen and excluded patients with a history of any vertebral abnormality or whose images revealed evidence of a vertebral abnormality or a congenital anomaly of any of the branches of the abdominal aorta. The primary outcome was the frequency distribution of the vertebral levels of the landmarks. The secondary outcomes were the intercorrelations of the vertebral levels of the landmarks and their relationships with age, sex, weight, height, and body mass index. The celiac artery originated at T11/T12–L1/L2, followed by the superior mesenteric artery at T12–L2, the paired renal arteries at T12/L1–L2/L3, the inferior mesenteric artery at L2–L4, and the common iliac arteries at L3–L5. The vertebral levels of the landmarks were positively intercorrelated and stronger between proximate pairs. In addition, the vertebral levels of the landmarks were related to age, but not sex, weight, height, or body mass index. The intercorrelations suggest that a considerable proportion of the variation is accounted for by ‘trickle-down’ variation; variation in the vertebral level of a proximal landmark results in variation in the vertebral level of the immediate distal landmark. The overarching parameter remains unidentified.
Go to :

Keywords: Abdominal aorta, Anatomy, Anatomic variation, Anatomic landmarks, Computed tomography angiography
Introduction
The aorta is the largest blood vessel in the human body. It originates in the thorax and terminates in the abdomen, crossing from one cavity to the other through a hiatus in the diaphragm. The abdominal portion of the aorta, or the abdominal aorta, gives off anterior, lateral, posterior, and terminal branches: The anterior branches are the celiac, superior mesenteric, inferior mesenteric, and testicular or ovarian arteries; the lateral branches are the middle suprarenal, renal, and inferior phrenic arteries; the posterior branches are the lumbar and median sacral arteries; and, the terminal branches are the common iliac arteries [
1].
Anatomists use the 33 vertebrae to reference the relative position of internal landmarks. The vertebral levels of the origins of the abdominal aorta branches are especially convenient to measure because the abdominal aorta runs anterior to the vertebral column. Indeed, many authors have documented the vertebral levels of the landmarks, which vary between individuals. Many factors may explain the variation. The usual suspects are age, sex, ethnicity, weight, height, and body mass index [
2-
4]. However, some questions remain unanswered. The interrelationships of the vertebral levels of the landmarks are unclear, and the relationships between the factors and the vertebral levels of the landmarks are not well substantiated. Therefore, our aim was to investigate the variation in the vertebral levels of six landmarks: the origins of the celiac artery, superior and inferior mesenteric arteries, paired renal arteries, and common iliac arteries.
Go to :

Materials and Methods
We conducted a retrospective imaging study in the Department of Radiology (Prince Hamzah Hospital) between January and March 2019. We drew our sample of participants from the picture archiving and imaging system of the department. We consecutively included adult patients (≥18 years of age) who had undergone computed tomography angiography of the abdomen using a 128-slice scanner. The images had been captured 15 seconds postinjection (75 ml iohexol 350 [4 ml/s] and 50 ml saline chaser [5 ml/s]). We excluded patients with a history of any vertebral abnormality or whose images revealed evidence of a vertebral abnormality or a congenital anomaly of the abdominal aorta branches. A member of our study team, who is a diagnostic radiologist with 10 years of experience, reviewed the computed tomography images of eligible patients and determined the vertebral levels of the origins of the celiac artery, superior and inferior mesenteric arteries, paired renal arteries, and common iliac arteries (
Fig. 1). We also retrieved the age, sex, weight, and height of each patient from the system. We calculated the body mass index using the standard formula (kg.m
−2). The primary outcome was the frequency distribution of the vertebral levels of the landmarks. The secondary outcomes were the intercorrelations of the vertebral levels of the landmarks and their relationships with age, sex, weight, height, and body mass index. The study protocol was approved by the ethics committee of the Prince Hamzah Hospital (6/2/2019/2020).
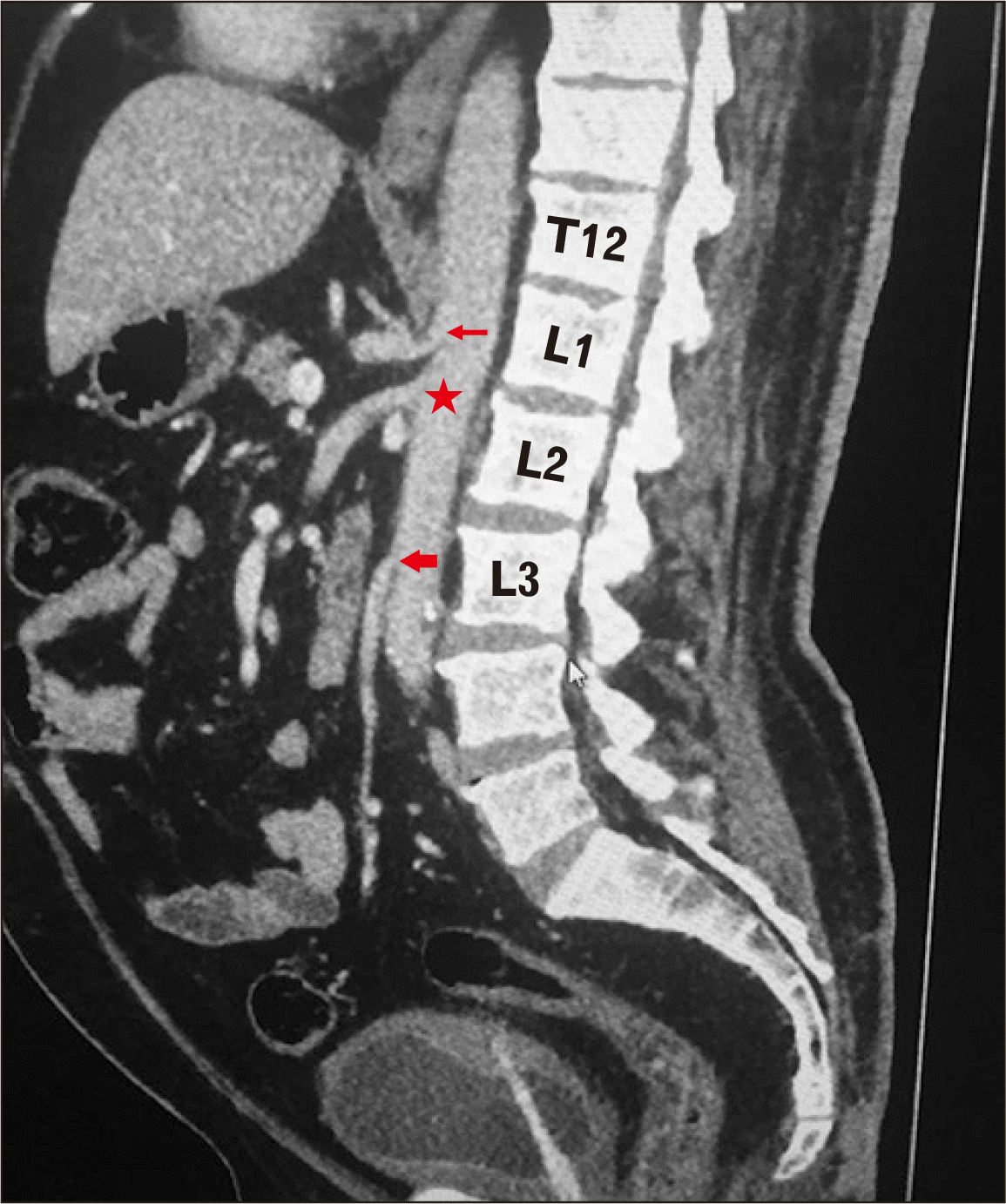 | Fig. 1Computed tomography angiography image showing the celiac (narrow red arrow), superior mesenteric (red star), and inferior mesenteric (wide red arrow) arteries. 
|
Statistical methods
We used R (version 3.6.1; R Development Core Team, 2019;
http://www.r-project.org) to perform power and data analyses. We calculated the minimum sample size for a test of correlation under the following assumptions: ρ=0.3, a=0.05, β=0.05. We also calculated the minimum sample size for K. Pearson’s χ
2 test under the following assumptions: w=0.3,
df=4, a=0.05, β=0.05. The minimum sample sizes were 138 and 207 participants, respectively, so the latter was our target. We summarized categorical data as n (%) and continuous data as mean (SD). We calculated Kendall’s τ
B and the estimated
P-value of the z test to measure the intercorrelations between the vertebral levels of the branch origins. To measure the uncertainty of each point estimate of Kendall’s τ
B, we computed the standard error of the statistic from 1,000 ordinary nonparametric bootstrap replicates, followed by the bias-corrected and accelerated confidence interval. We used the same to measure the correlations of the vertebral levels of the branch origins with age, weight, height, and body mass index. To measure the association of the vertebral levels of the branch origins with sex, we calculated K. Pearson’s χ
2. To account for some expected cell frequencies between one and five, we applied E. Pearson’s
N−1 correction to K. Pearson’s χ
2 and calculated the
P-value of the corrected statistic [
5]. To account for multiple testing, we corrected the
P-values using the Benjamini–Hochberg procedure. We present numerical data according to the recommendations of Cole [
6].
Go to :

Results
We included 227 participants in the final analysis. No data were missing. The mean age, weight, height, and body mass index of the participants were 54±15 years, 78±17 kg, 166±8 cm, and 28±6 kg.m−2 respectively. One hundred and six were female (46.7%) and 121 were male (53.3%).
We studied the vertebral levels of the origins of the celiac artery, superior and inferior mesenteric arteries, paired renal arteries, and common iliac arteries. The origins of all the branches varied between exactly two vertebral levels (
Fig. 2). The celiac artery originated at T11/T12–L1/L2, followed by the superior mesenteric artery at T12–L2, the paired renal arteries at T12/L1–L2/L3, the inferior mesenteric artery at L2–L4, and the common iliac arteries at L3–L5. We present the frequency distribution of the vertebral levels of the origins in
Table 1.
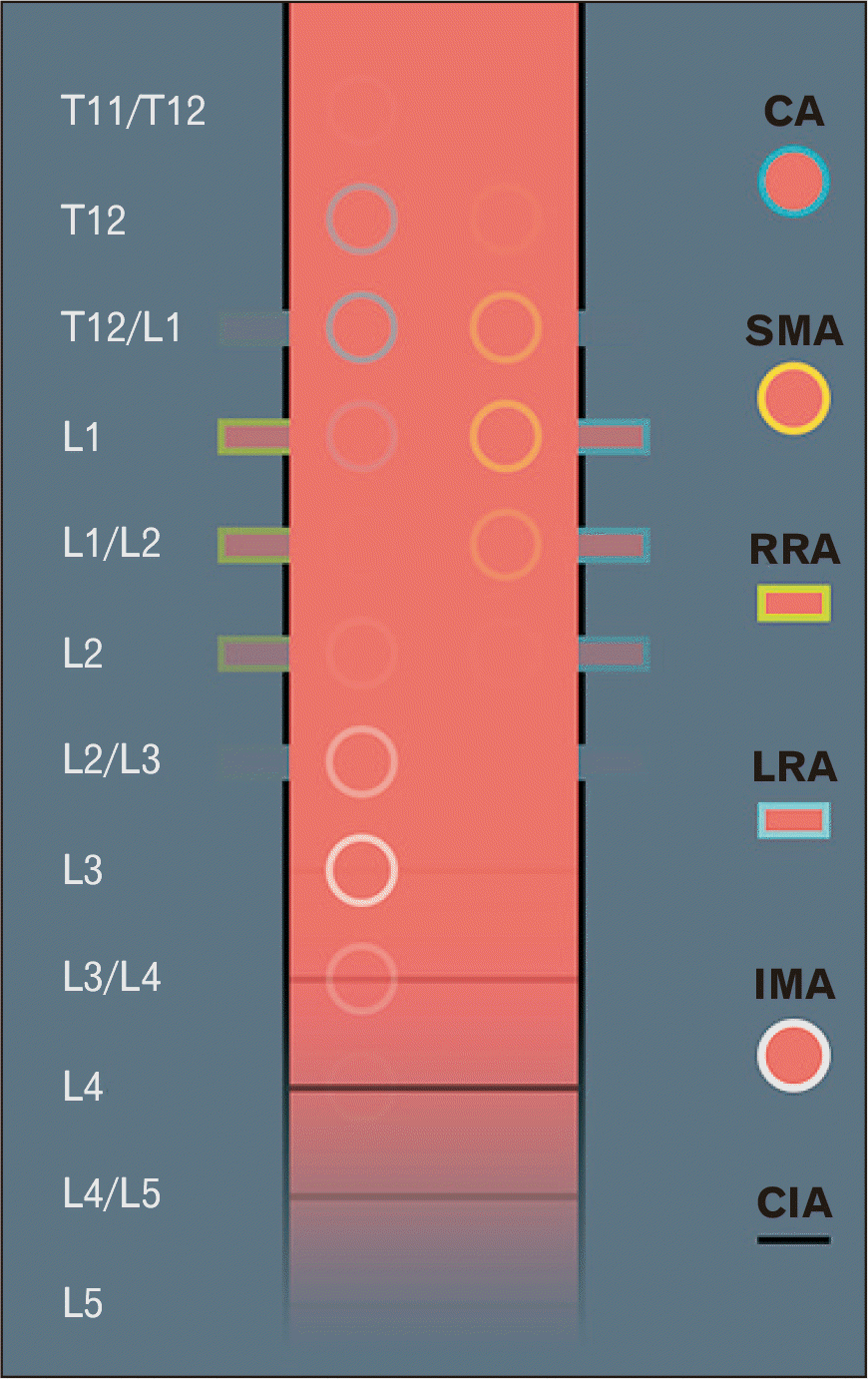 | Fig. 2The vertebral levels of the origins of the abdominal aorta branches (n=227 participants). We used the proportions of incidence in our study to determine the degree of opacity of the visual representation of the variants. CA, celiac artery; CIA, common iliac arteries; IMA, inferior mesenteric artery; LRA, left renal artery; RRA, right renal artery; SMA, superior mesenteric artery. 
|
Table 1
The vertebral levels of the origins of six abdominal aorta branches (n=227)
|
Vertebral level |
Celiac artery |
Superior mesenteric artery |
Right renal artery |
Left renal artery |
Inferior mesenteric artery |
Common iliac arteries |
|
T11/T12 |
10 (4.4) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
T12 |
80 (35.2) |
9 (4.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
|
T12/L1 |
96 (42.3) |
69 (30.4) |
9 (4.0) |
3 (1.3) |
0 (0.0) |
0 (0.0) |
|
L1 |
39 (17.2) |
105 (46.3) |
93 (41.0) |
92 (40.5) |
0 (0.0) |
0 (0.0) |
|
L1/L2 |
2 (0.9) |
41 (18.1) |
82 (36.6) |
78 (34.4) |
0 (0.0) |
0 (0.0) |
|
L2 |
0 (0.0) |
3 (1.3) |
39 (17.2) |
50 (22.0) |
10 (4.4) |
0 (0.0) |
|
L2/L3 |
0 (0.0) |
0 (0.0) |
4 (1.8) |
4 (1.8) |
58 (25.6) |
0 (0.0) |
|
L3 |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
122 (53.7) |
9 (4.0) |
|
L3/L4 |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
32 (14.1) |
40 (17.6) |
|
L4 |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
5 (2.2) |
128 (56.4) |
|
L4/L5 |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
41 (18.1) |
|
L5 |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
0 (0.0) |
9 (4.0) |

The territories of the origins of the celiac and inferior mesenteric arteries were completely distinct; the origin of the celiac artery did not occur below L1/L2, and the origin of the inferior mesenteric did not occur above L2. The territories of the origins of the superior and inferior mesenteric arteries overlapped at L2 but were otherwise distinct. In relation to the left renal artery, the right renal artery originated at a higher level in 34 cases (15.0%), the same level in 183 cases (80.6%), and a lower level in 10 cases (4.4%).
The vertebral levels of the origins of all branches were positively and statistically significantly intercorrelated. The intercorrelations were stronger between proximate branches than distant branches. We present the correlation matrix in
Table 2. In addition, the vertebral levels of the origins of all branches were related to age, but not sex, weight, height, or body mass index (
Table 3,
Table 4).
Table 2
Intercorrelation of the vertebral levels of the origins of six abdominal aorta branches (n=227)
|
Artery |
Celiac artery |
Superior mesenteric artery |
Right renal artery |
Left renal artery |
Inferior mesenteric artery |
|
Superior mesenteric artery |
0.88 (0.84–0.92); <0.001 |
|
|
|
|
|
Right renal artery |
0.65 (0.57–0.73); <0.001 |
0.67 (0.59–0.74); <0.001 |
|
|
|
|
Left renal artery |
0.67 (0.59–0.73); <0.001 |
0.68 (0.59–0.74); <0.001 |
0.82 (0.76–0.87); <0.001 |
|
|
|
Inferior mesenteric artery |
0.44 (0.34–0.53); <0.001 |
0.50 (0.41–0.58); <0.001 |
0.52 (0.42–0.59); <0.001 |
0.51 (0.40–0.58); <0.001 |
|
|
Common iliac arteries |
0.41 (0.31–0.51); <0.001 |
0.46 (0.37–0.53); <0.001 |
0.47 (0.38–0.55); <0.001 |
0.44 (0.34–0.52); <0.001 |
0.68 (0.59–0.75); <0.001 |

Table 3
Correlations of the vertebral levels of the origins of six abdominal aorta branches with age, weight, height, and body mass index (n=227)
|
Measure |
Celiac artery |
Superior mesenteric artery |
Right renal artery |
Left renal artery |
Inferior mesenteric artery |
Common iliac arteries |
|
Age |
0.21 (0.11–0.31);
<0.001 |
0.22 (0.11–0.31);
<0.001 |
0.27 (0.17–0.37);
<0.001 |
0.26 (0.15–0.35);
<0.001 |
0.27 (0.17–0.36);
<0.001 |
0.26 (0.16–0.35);
<0.001 |
|
Weight |
0.03 (−0.07 to 0.14);
0.6 |
0.01 (−0.10 to 0.12);
0.9 |
0.03 (−0.06 to 0.14);
0.7 |
0.02 (−0.08 to 0.12);
0.8 |
0.06 (−0.04 to 0.17);
0.4 |
0.09 (−0.02 to 0.19);
0.2 |
|
Height |
0.09 (−0.02 to 0.20);
0.2 |
0.09 (−0.02 to 0.20);
0.2 |
0.07 (−0.03 to 0.17);
0.3 |
0.01 (−0.10 to 0.11);
0.9 |
−0.04 (−0.16 to 0.06);
0.6 |
0.04 (−0.07 to 0.14);
0.6 |
|
Body mass index |
0.01 (−0.10 to 0.12);
0.9 |
−0.01 (−0.12 to 0.09);
0.9 |
0.01 (−0.09 to 0.11);
0.9 |
0.02 (−0.07 to 0.12);
0.8 |
0.09 (−0.03 to 0.19);
0.2 |
0.07 (−0.03 to 0.17);
0.3 |

Table 4
Associations of the vertebral levels of the origins of six abdominal aorta branches with sex (n=227)
|
Branch |
P-value |
|
Celiac artery |
0.3 |
|
Superior mesenteric artery |
0.4 |
|
Right renal artery |
0.6 |
|
Left renal artery |
0.2 |
|
Inferior mesenteric artery |
0.6 |
|
Common iliac arteries |
0.6 |

Go to :

Discussion
We conducted a retrospective imaging study of the variation in the vertebral levels of the origins of the abdominal aorta branches in a nonrandom sample of the Jordanian population. We found that the territories of all the landmarks spanned exactly two vertebral levels. In addition, we found that the vertebral levels of the landmarks were positively and statistically significantly intercorrelated and related to age, but not sex, weight, height, or body mass index.
The vertebral levels of the origins of the abdominal aorta branches have long since been reported in cadaveric studies of samples drawn from diverse populations. Our results are consistent with seminal reports from Canada, Japan, and Germany [
7]. Recent imaging studies, based in India and Greece, have also reported similar results [
2,
8]. Therefore, ethnicity does not appear to be a significant contributor to the variation in the vertebral levels of the landmarks. We went further and measured the intercorrelations between the vertebral levels of the origins. Our findings suggest that variation in the most proximal branch of the abdominal aorta ‘trickles down’ to the distal branches. However, ‘trickle-down’ variation explained a part, but not all of the variation.
We studied the relationships of the variation with age, sex, weight, height, and body mass index. Separate authors have long since reported a distal shift of abdominal structures, including the branches of the abdominal aorta, with age [
7]. The whole length of the aorta increases with age because elastin fibers are replaced with collagen fibers [
9]. Our results are consistent with the foregoing references; we found consistent correlations between age and the vertebral levels of the origins. In contrast, three teams of authors recently reproduced the correlation for some arteries but not others, or none at all [
2-
4]. The sample sizes in all three studies were less than half of our sample size, so differences in power may explain the inconsistency. In addition, we found no association between sex and the variation, which is consistent with previous reports [
2-
4]. Finally, one team reported a relationship between body mass index and the vertebral level of the origin of the celiac artery but not the other arteries [
3]. In contrast, we found no correlation between weight, height, and body mass index and the vertebral level of the origin of any branch.
Our study design limits the interpretability of our results. First, our study was not longitudinal, so we cannot substantiate the cause of the correlations between age and the vertebral levels of the origins of the abdominal aorta branches. The correlations may result from generational variation due to a factor or a set of factors we did not consider. Second, we used a nonrandom sampling technique, so our estimates likely do not represent the Jordanian population. Third, our study was underpowered to detect weak relationships, so our results must be interpreted with caution. Nevertheless, the power of our study is higher than the other studies referenced in our report. In addition, we show that a considerable part of the variation in the vertebral levels of the origins is due to intercorrelations, which is a new finding with possible implications. For example, variation in the position of a yet unidentified proximal landmark may explain the variation in the vertebral levels of the origins of the abdominal aorta branches (‘trickle-down’ variation).
In conclusion, the intercorrelations suggest that a considerable proportion of the variation in the vertebral levels of the origins of the abdominal aorta branches is accounted for by ‘trickle-down’ variation; variation in the vertebral level of a proximal landmark results in variation in the vertebral level of the immediate distal landmark. The overarching parameter remains unidentified.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download