Introduction
Materials and Methods
Chemicals and drugs
Treatment protocol
Group 1: considered as the negative control receiving saline intraperitoneal (2 ml/kg i.p.) once/day for 7, 14, 21 days consecutively, and represented as a negative control group
Group 2: rats were given meloxicam (2 mg/kg i.p) for 7, 14, 21 days [11].
Group 3: a single DOX dose (15 mg/kg i.p.) was injected to rats [15].
Group 4: a single DOX dose was injected to rats (i.p.) followed by Vit D3 orally (Devit-3, 50,000 IU/15 ml) daily for 7, 14, 21 days. Vit D3 had been given into the mouth by dropper (200 IU/day/rat) [4]
Group 5: a single DOX dose was injected to rats (i.p.) followed by meloxicam (2 mg/kg i.p.) for 7, 14, 21 days [11].
Urinary tests
Sample collection and assessment of renal functions
Evaluation of reduced glutathione and renal lipid peroxides
Histological assessment
Immunohistochemical examination
Morphometric analysis
Glomerular geometry
Glomerular sclerosis and tubulointerstitial damage indices
Statistical analysis
Results
Effect on urinary protein
 | Fig. 1Total urinary protein (A), serum creatinine (B), MDA level in renal tissue (C), and GSH in renal tissue (D) for groups with saline treated (●), meloxicam (■), DOX (▲), DOX+Vit D3 (▽) and DOX+meloxicam (□) treated groups graphed versus time. Values are presented as means±SD. DOX, doxorubicin; GSH, glutathione; MDA, malondialdehyde; Vit D3, vitamin D3. *P, significant difference between combined DOX+Vit D3 vs. combined DOX+meloxicam treated group; **P, significant in contrast to other groups. |
Effect on serum creatinine
Effect on lipid peroxidation
Time course variations of renal glutathione
Table 1
| Group | 1st wk (%) | 2nd wk (%) | 3rd wk (%) |
|---|---|---|---|
| Saline (control) | 100a) | 100a) | 100a |
| Meloxicam | 110.93±6a) | 108.52±5a) | 100.52±8a) |
| DOX | 69.95±5b) | 60.23±4b) | 46.91±2b) |
| DOX+Vit D3 | 90.71±7c) | 85.23±6c) | 68.04±4c) |
| DOX+meloxicam | 93.99±7c) | 92.35±5d) | 82.99±3d) |
Morphometric results
Table 2
| Group | 1st wk | 2nd wk | 3rd wk | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|
||||||||||
| Mean volume of individual glomeruli (×105 μm3) | Glomerular surface area (×103 μm2) | Peripheral urinary surface area (×103 μm2) | Average number of nuclei per glomerulus | Mean volume of individual glomeruli (×105 μm3) | Glomerular surface area (×103 μm2) | Peripheral urinary surface area (×103 μm2) | Average number of nuclei per glomerulus | Mean volume of individual glomeruli (×105 μm3) | Glomerular surface area (×103 μm2) | Peripheral urinary surface area (×103 μm2) | Mean number of nuclei per glomerulus | |
| Saline (control) | 3.95±0.59a,c) | 6.10±0.31a,c) | 1.52±0.77a,c) | 49.00±9.90a,b) | 3.72±0.56a) | 6.01±3.90a,c) | 1.70±0.10a,c) | 43.50±3.43a,c) | 3.47±0.84a,c) | 6.37±1.02a) | 2.14±0.35a,c) | 47.33±1.53a) |
| meloxicam | 4.42±1.61c) | 6.27±4.05c) | 1.40±0.15c) | 55.50±0.71a) | 4.12±0.20a,c) | 6.32±2.45a,c) | 1.73±0.97a,c) | 42.00±3.00a,c) | 5.17±0.12a) | 7.64±1.14a) | 2.12±0.75a,c) | 48.50±4.95a) |
| DOX | 1.90±0.96b) | 6.76±4.03a,c) | 2.27±1.65b,c) | 39.75±7.32b,c) | 1.65±0.13b,c) | 6.86±2.72a,c) | 2.93±1.02a) | 37.00±9.61a,c) | 1.13±0.13b) | 4.67±2.42b) | 3.33±1.26a) | 32.00±32.00b,c) |
| DOX+Vit D3 | 3.25±2.35a) | 5.00±2.74a) | 1.53±0.96a,b) | 45.67±1.55b,c) | 3.27±0.23a) | 5.07±2.01b,c) | 1.18±0.70b,c) | 32.50±0.71a) | 3.60±0.22a,c) | 5.75±2.95a) | 2.27±0.98a,c) | 42.66±4.72a) |
| DOX+meloxicam | 3.92±0.28a) | 5.65±2.70a) | 1.22±0.78a) | 55.20±5.81a) | 3.90±0.13b,c) | 5.70±1.71b) | 1.08±0.72b,c) | 53.50±3.32b,c) | 3.82±0.16a,c) | 6.16±1.50a) | 1.07±0.40b,c) | 43.50±2.12a) |
Table 3
| Group | Glomerular sclerosis | Tubulointerstitial damage | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 1st wk | 2nd wk | 3rd wk | 1st wk | 2nd w wk | 3rd wk | |
| Saline (control) | 0.0±0.0a,c) | 0.0±0.0a) | 0.0±0.0a) | 0.0±0.0a) | 0.0±0.0a) | 0.0±0.0a) |
| Meloxicam | 0.0±0.0a) | 0.0±0.0a) | 0.0±0.0a) | 0.0±0.0a) | 0.0±0.0a) | 0.0±0.0a) |
| DOX | 1.13±0.83b) | 1.67±0.43b) | 2.67±0.64b) | 1.50±0.38b) | 2.40±0.40b) | 3.60±0.45b) |
| DOX+Vit D3 | 0.75±0.26a) | 1.63±0.51b) | 2.30±0.15b,c) | 0.90±0.23b,c) | 1.43±0.20c) | 3.25±0.50b) |
| DOX+meloxicam | 0.25±0.10c) | 0.60±0.29a) | 1.50±0.18c) | 0.45±0.16a,c) | 0.90±0.18c) | 1.86±0.69c) |
Histopathological assessment
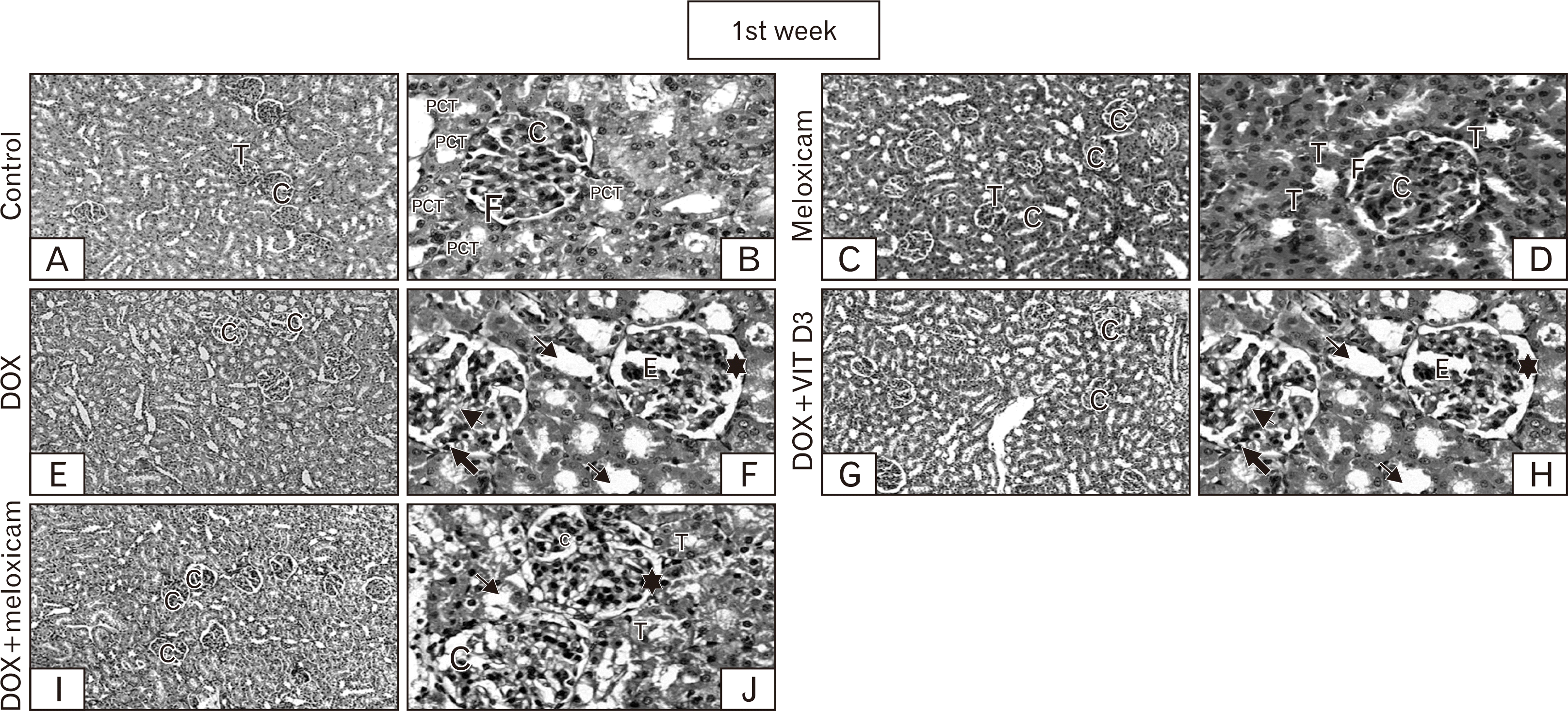 | Fig. 2Representative of cortical kidney sections stained by H&E from rats representing groups of control, meloxicam, DOX, DOX+Vit D3, and DOX+meloxicam after 1st week. Control sections revealed the normal histological architecture, renal T and renal C (A, ×100), F and PCT (B, ×400). Meloxicam revealed no changes (C, ×100). The renal glomeruli and tubules appear to great extent normal (D, ×400). DOX is showing decrease number of renal C in contrast to other groups (E, ×100), glomerular E, synechia of the glomerular tuft with Bowman’s capsule (➨), mesangial matrix increment (➤), and dilation of the F (🟌). Some cells of PCT loss its brush borders and some nuclei appear pyknotic (→) (F, ×400). Conversely, combination group revealed rare disruption of some renal C (G, I, ×100). Less dilation of F (🟌). Most of PCT appears normal (T) lower number of cells of PCT showed loss of brush border and pyknosis (→) compared to the DOX treated group (H, J, ×400). C, corpuscles; DOX, doxorubicin; E, edema; F, filtration space; PCT, proximal convoluted tubules; T, tubules; Vit D3, vitamin D3. |
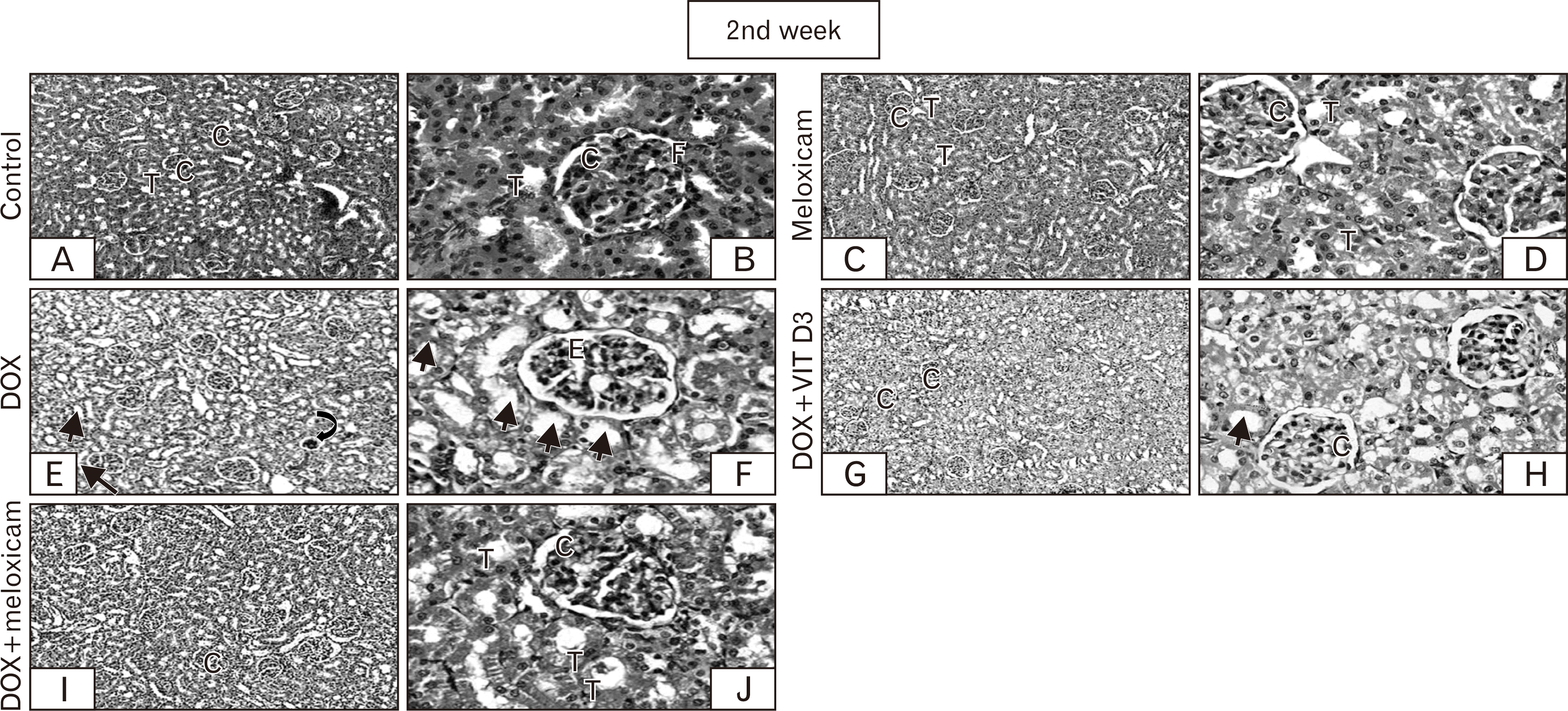 | Fig. 3After 2nd week: Sections of control group demonstrating the normal histological architecture, renal T, renal C (A, ×100), and F (B, ×400). No difference is shown in meloxicam (C, ×100). The renal glomeruli and tubules appear normal to great extent (D, ×400). DOX is showing shrinkage of renal C (⮏) compared to other groups (E, ×100), some showing glomerular E and most cells of PCT loss its brush borders and some nuclei appear psychotic (→) (F, ×400). However, combination group exhibiting less disruption of some renal C (G, I, ×100). Most of PCT appears normal (T) lower number of cells of PCT showed loss of brush border and pyknosis (→) compared to the DOX treated group (H, J, ×400), with better picture in DOX+meloxicam compared with DOX+Vit D3 group. C, corpuscles; DOX, doxorubicin; E, edema; F, filtration space; T, tubules; Vit D3, vitamin D3. |
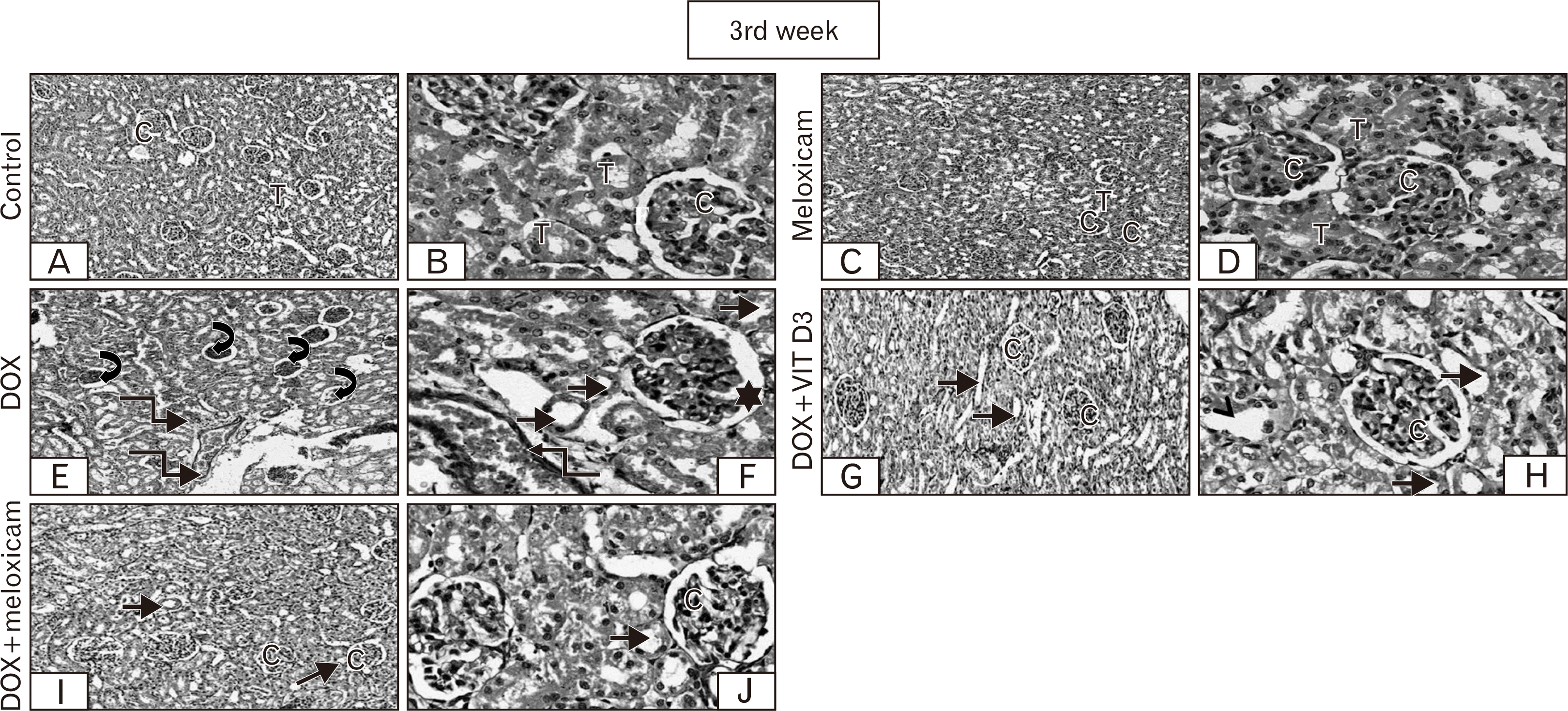 | Fig. 4After 3rd week Sections of control group exhibiting the normal histological architecture, renal C, renal T (A, ×100), and F (B, ×400). Meloxicam is showing no difference from the normal (C, ×100). The renal glomeruli and tubules appear to great extent normal (D, ×400). DOX is showing prominent shrinkage of renal C (⮏) compared to other groups and vascular congestion (↯) (E×100), and dilation of the F (🟌). Most cells of PCT loss its brush borders, dilated and some nuclei appear psychotic (→) (F×400). However, combination group showing less distortion of renal C (G, I, ×100). Most prominent finding is dilation of T and loss of brush borders (→) compared to the control group (H, J, ×400), with better picture in DOX+meloxicam compared with DOX+Vit D3 group. C, corpuscle; DOX, doxorubicin; F, filtration space; PCT, proximal convoluted tubules; T, tubules. |
Immunohistochemical results
 | Fig. 5Representatives of renal cortical tissue stained by TNF-α of: (A–C), control and meloxicam groups (D–F) after 1st, 2nd, and 3rd weeks, respectively displaying negative expression. DOX treated animals displayed intensive expression (G–I) after (1st, 2nd, and 3rd weeks respectively) in the renal glomeruli (→) and renal tubules (➤). Combined DOX-Vit D3 treated rats displayed moderate expression (J–L) after (1st, 2nd, and 3rd weeks respectively) within the glomeruli (→) while mild expression in the renal tubules (➤) .DOX/meloxicam treated groups demonstrated considerable improvement with mild expression (M–O) after (1st, 2nd, and 3rd weeks respectively) in the renal glomeruli (→) and renal tubules (➤). The expression is predominantly cytoplasmic, but with few nuclear expression. Immunohistochemistry counter stained with H&E, ×400. DOX, doxorubicin; TNF-α, tumor necrosis factor-alpha; Vit D3, vitamin D3. |
Table 4
| Group | Glomerular expression | Tubulointerstitial expression | ||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| 1st wk | 2nd wk | 3rd wk | 1st wk | 2nd wk | 3rd wk | |
| Saline (control) | 1.75±0.50a) | 1.00±1.00a) | 0.50±0.21a) | 1.00±0.00a) | 1.00±0.00a) | 0.45±0.25a) |
| Meloxicam | 0.50±0.21a) | 1.00±1.00a) | 1.25±0.50a,c) | 0.80±0.30a) | 1.10±0.32a) | 1.11±0.29a) |
| DOX | 4.67±2.88b) | 5.60±2.19b) | 6.00±2.19b) | 3.13±1.55b) | 3.38±2.42b,c) | 4.37±1.85b) |
| DOX+Vit D3 | 3.57±1.72c) | 4.50±1.91b) | 4.67±2.30b,c) | 1.95±1.16a) | 2.21±1.22a,c) | 2.68±1.40a) |
| DOX+meloxicam | 1.67±0.57a) | 1.67±1.15a) | 2.50±1.22a) | 1.93±0.91a) | 2.00±0.33a,c) | 2.06±0.74a) |




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download