1. Hong SB, Jung KY. Basic electrophysiology of the electroencephalography. J Korean Neurol Assoc. 2003; 21:225–238.
2. Buzsáki G, Anastassiou CA, Koch C. The origin of extracellular fields and currents--EEG, ECoG, LFP and spikes. Nat Rev Neurosci. 2012; 13:407–420.
3. Buzsáki G, Draguhn A. Neuronal oscillations in cortical networks. Science. 2004; 304:1926–1929.

4. Jensen O, Spaak E, Zumer JM. Human brain oscillations: from physiological mechanisms to analysis and cognition. In : Supek S, Aine CJ, editors. Magnetoencephalography: from signals to dynamic cortical networks. New York: Springer International Publishing;2019. p. 1–46.
5. Jung KY. Characteristics and analysis methods of EEG signals. In : . Art and application of EEG analysis: from basic to clinical research. Seoul: Daehan Medical Book Publishing;2017. p. 124–145.
6. Stam CJ, van Straaten EC. The organization of physiological brain networks. Clin Neurophysiol. 2012; 123:1067–1087.

7. Stam CJ. Modern network science of neurological disorders. Nat Rev Neurosci. 2014; 15:683–695.

8. Delorme A, Makeig S. EEGLAB: an open source toolbox for analysis of single-trial EEG dynamics including independent component analysis. J Neurosci Methods. 2004; 134:9–21.

9. Makeig S, Bell AJ, Jung TP, Sejnowski TJ. Independent component analysis of electroencephalographic data. In : Mozer MC, Jordan MI, Petsche T, editors. Advances in Neural Information Processing Systems 9 (NIPS 1996). London: MIT Press;1996. p. 145–151.
10. Jung TP, Makeig S, Humphries C, Lee TW, McKeown MJ, Iragui V, et al. Removing electroencephalographic artifacts by blind source separation. Psychophysiology. 2000; 37:163–178.

11. Crespo-Garcia M, Atienza M, Cantero JL. Muscle artifact removal from human sleep EEG by using independent component analysis. Ann Biomed Eng. 2008; 36:467–475.

12. Polat K, Güneş S. Classification of epileptiform EEG using a hybrid system based on decision tree classifier and fast Fourier transform. Appl Math Comput. 2007; 187:1017–1026.

13. Mormann F, Lehnertz K, David P, Elger C. Mean phase coherence as a measure for phase synchronization and its application to the EEG of epilepsy patients. Physica D: Nonlinear Phenomena. 2000; 144:358–369.

14. Lachaux JP, Rodriguez E, Martinerie J, Varela FJ. Measuring phase synchrony in brain signals. Hum Brain Mapp. 1999; 8:194–208.

15. Doesburg SM, Emberson LL, Rahi A, Cameron D, Ward LM. Asynchrony from synchrony: long-range gamma-band neural synchrony accompanies perception of audiovisual speech asynchrony. Exp Brain Res. 2008; 185:11–20.

16. Vinck M, Oostenveld R, van Wingerden M, Battaglia F, Pennartz CM. An improved index of phase-synchronization for electrophysiological data in the presence of volume-conduction, noise and sample-size bias. Neuroimage. 2011; 55:1548–1565.

17. Bullmore E, Sporns O. Complex brain networks: graph theoretical analysis of structural and functional systems. Nat Rev Neurosci. 2009; 10:186–198.

18. Stam CJ, Reijneveld JC. Graph theoretical analysis of complex networks in the brain. Nonlinear Biomed Phys. 2007; 1:3.

19. Fisher RS, Acevedo C, Arzimanoglou A, Bogacz A, Cross JH, Elger CE, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia. 2014; 55:475–482.

20. Kramer MA, Cash SS. Epilepsy as a disorder of cortical network organization. Neuroscientist. 2012; 18:360–372.

21. Bartolomei F, Wendling F, Régis J, Gavaret M, Guye M, Chauvel P. Pre-ictal synchronicity in limbic networks of mesial temporal lobe epilepsy. Epilepsy Res. 2004; 61:89–104.

22. Bettus G, Wendling F, Guye M, Valton L, Régis J, Chauvel P, et al. Enhanced EEG functional connectivity in mesial temporal lobe epilepsy. Epilepsy Res. 2008; 81:58–68.

23. Bernhardt B, Hong SJ, Bernasconi A, Bernasconi N. Imaging structural and functional brain networks in temporal lobe epilepsy. Front Hum Neurosci. 2013; 7:624.

24. Bonilha L, Helpern JA, Sainju R, Nesland T, Edwards JC, Glazier SS, et al. Presurgical connectome and postsurgical seizure control in temporal lobe epilepsy. Neurology. 2013; 81:1704–1710.

25. Lagarde S, Roehri N, Lambert I, Trebuchon A, McGonigal A, Carron R, et al. Interictal stereotactic-EEG functional connectivity in refractory focal epilepsies. Brain. 2018; 141:2966–2980.

26. Horstmann MT, Bialonski S, Noennig N, Mai H, Prusseit J, Wellmer J, et al. State dependent properties of epileptic brain networks: comparative graph-theoretical analyses of simultaneously recorded EEG and MEG. Clin Neurophysiol. 2010; 121:172–185.

27. Quraan MA, McCormick C, Cohn M, Valiante TA, McAndrews MP. Altered resting state brain dynamics in temporal lobe epilepsy can be observed in spectral power, functional connectivity and graph theory metrics. PLoS One. 2013; 8:e68609.

28. Wilke C, Worrell G, He B. Graph analysis of epileptogenic networks in human partial epilepsy. Epilepsia. 2011; 52:84–93.

29. Reitz C, Brayne C, Mayeux R. Epidemiology of Alzheimer disease. Nat Rev Neurol. 2011; 7:137–152.

30. Jeong J. EEG dynamics in patients with Alzheimer’s disease. Clin Neurophysiol. 2004; 115:1490–1505.

31. Stam CJ, van der Made Y, Pijnenburg YA, Scheltens P. EEG synchronization in mild cognitive impairment and Alzheimer’s disease. Acta Neurol Scand. 2003; 108:90–96.

32. Stam CJ, Montez T, Jones BF, Rombouts SA, van der Made Y, Pijnenburg YA, et al. Disturbed fluctuations of resting state EEG synchronization in Alzheimer’s disease. Clin Neurophysiol. 2005; 116:708–715.

33. Stam CJ, Jones BF, Nolte G, Breakspear M, Scheltens P. Smallworld networks and functional connectivity in Alzheimer’s disease. Cereb Cortex. 2007; 17:92–99.

34. Stam CJ, de Haan W, Daffertshofer A, Jones BF, Manshanden I, van Cappellen van Walsum AM, et al. Graph theoretical analysis of magnetoencephalographic functional connectivity in Alzheimer’s disease. Brain. 2009; 132(Pt 1):213–224.

35. McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, et al. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology. 2017; 89:88–100.
36. Briel RC, McKeith IG, Barker WA, Hewitt Y, Perry RH, Ince PG, et al. EEG findings in dementia with Lewy bodies and Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1999; 66:401–403.

37. Bonanni L, Thomas A, Tiraboschi P, Perfetti B, Varanese S, Onofrj M. EEG comparisons in early Alzheimer’s disease, dementia with Lewy bodies and Parkinson’s disease with dementia patients with a 2-year follow-up. Brain. 2008; 131(Pt 3):690–705.

38. van Dellen E, de Waal H, van der Flier WM, Lemstra AW, Slooter AJ, Smits LL, et al. Loss of EEG network efficiency is related to cognitive impairment in dementia with lewy bodies. Mov Disord. 2015; 30:1785–1793.
39. McCann H, Stevens CH, Cartwright H, Halliday GM. α-Synucleinopathy phenotypes. Parkinsonism Relat Disord. 2014; 20 Suppl 1:S62–S67.

40. You S, Jeon SM, Cho YW. Rapid eye movement sleep behavior disorder. J Sleep Med. 2018; 15:1–7.

41. Postuma RB, Iranzo A, Hu M, Högl B, Boeve BF, Manni R, et al. Risk and predictors of dementia and parkinsonism in idiopathic REM sleep behaviour disorder: a multicentre study. Brain. 2019; 142:744–759.
42. Fantini ML, Gagnon JF, Petit D, Rompré S, Décary A, Carrier J, et al. Slowing of electroencephalogram in rapid eye movement sleep behavior disorder. Ann Neurol. 2003; 53:774–780.
43. Rodrigues Brazète J, Gagnon JF, Postuma RB, Bertrand JA, Petit D, Montplaisir J. Electroencephalogram slowing predicts neurodegeneration in rapid eye movement sleep behavior disorder. Neurobiol Aging. 2016; 37:74–81.

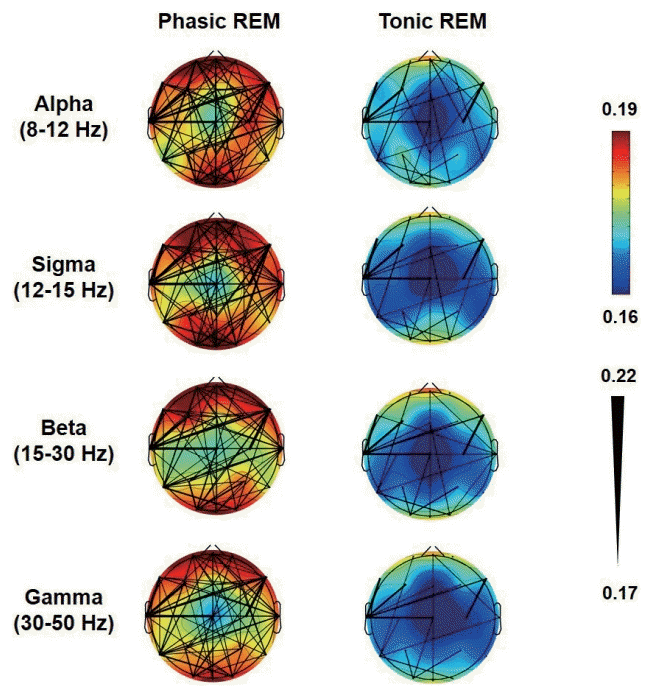
44. Sunwoo JS, Lee S, Kim JH, Lim JA, Kim TJ, Byun JI, et al. Altered functional connectivity in idiopathic rapid eye movement sleep behavior disorder: a resting-state EEG study. Sleep. 2017; Apr. 18. [Epub]. DOI:10.1093/sleep/zsx058.

45. Olde Dubbelink KT, Stoffers D, Deijen JB, Twisk JW, Stam CJ, Hillebrand A, et al. Resting-state functional connectivity as a marker of disease progression in Parkinson’s disease: a longitudinal MEG study. Neuroimage Clin. 2013; 2:612–619.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download