Abstract
Vestibular-evoked myogenic potentials (VEMPs) are useful for evaluating the vestibulocollic reflex arising mostly from the saccule and the vestibuloocular reflex originating from the utricle. VEMPs can vary with the characteristics of the applied stimuli and the effects of aging and diseases. VEMPs have been found to be useful for diagnosing superior canal dehiscence, but their usefulness for other clinical disorders remains unclear. This review discusses the principles of VEMP tests and summarizes the findings for VEMPs in common vestibular disorders.
The otolith organs comprise the saccule and utricle, which respond to linear accelerations of the head. Vestibular-evoked myogenic potentials (VEMPs) are useful for evaluating otolith function.1,2 Two subtypes of VEMPs are currently utilized: cervical and ocular (Fig. 1).1,2 Cervical VEMPs were introduced first,3 and they can be used to evaluate the neural pathway comprising the saccule, vestibular nerve, and nucleus, and the descending vestibulospinal tract innervating the cervical neck muscles.4 Cervical VEMPs are biphasic waves of positive and negative potentials observed at around 13 and 23 ms, respectively, after stimulation, which can be detected on the surface of the sternocleidomastoid muscle.3,4 Ocular VEMPs were introduced later,5,6 and they can be used to evaluate the neural pathway consisting of the utricle, vestibular nerve, and nucleus, and the medial longitudinal fasciculus, which ends in the inferior oblique muscle.5,6 Ocular VEMPs are also biphasic waves formed by negative and positive potentials observed at around 10 and 15 ms, respectively, after stimulation, and they can be measured at just below the midline of the inferior orbital wall.5,6 Cervical and ocular VEMPs have been evaluated in several vestibular disorders, and a few techniques have been developed for enhancing the diagnostic efficacy of VEMPs.
VEMPs are elicited using air-conducted (AC) clicks or tone bursts, bone-conducted (BC) vibrations induced by a bone-conduction vibrator or manual tapping, or galvanic electrical stimulation.1,2 All of these stimuli can deliver impulses to the otolith organs and induce movements of the hair cells, but the responses vary with the characteristics of the stimuli. Cervical VEMPs were initially evoked using click sounds, which include a wide range of audible frequencies.3 VEMPs can be evoked in subjects with complete sensorineural hearing loss, implying that AC sounds stimulate the otolith hair cells.3 In animal studies, the vestibular afferents responded to AC sounds between 500 Hz and 1 kHz, while the cochlear afferents responded widely across audible frequencies, and the response threshold was reported to be above 90 dB sound pressure level (SPL) for the vestibular afferents.7,8 Likewise, cervical VEMPs are best evoked in humans when using similar auditory frequencies and intensities.9 Therefore, in addition to click sounds, single tone bursts that selectively contain specific auditory frequencies are commonly used to evoke VEMPs; a typical single tone burst is applied at 500 Hz and 90-115 dB SPL.1,2 The best stimulus frequency to apply for ocular VEMPs is 1 kHz, which is higher than the optimal frequency of 500 Hz for cervical VEMPs;10 however, auditory stimuli at the same frequency auditory stimuli are commonly applied for both cervical and ocular VEMPs. Unlike AC stimuli, BC stimuli can be delivered symmetrically bilaterally by positioning the stimulus in the middle of the forehead.1,2 Therefore, BC stimuli may be a suitable alternative when the raw amplitudes of VEMPs cannot be corrected using muscular activity.
The basic parameters for VEMP tests are the latency and the peak-to-peak amplitude. These parameters can be interpreted by itself, but the asymmetry of the amplitude—calculated as the difference in amplitudes between the ears divided by the sum of the amplitudes in both ears—is more useful for interpretations.1,2 Normal limits for amplitude and asymmetry should be set for each individual laboratory. Although the weaker side is generally considered abnormal, there would be paradoxical enhancement of VEMPs in superior canal dehiscence (SCD) and the early stage of Meniere’s disease (MD).1,2
In cervical VEMP tests, the inhibitory potentials are measured in the sternocleidomastoid muscle. To ensure adequate muscle activation, subjects are instructed to lift their head while in the supine position1,2 or, while in a seated position, they rotate their head while pushing against the examiner’s hand or the inflated cuff of a sphygmomanometer.11 The amplitude of VEMPs increase in proportion with the contraction activity of the recorded muscle.3,12 Because the interaural difference in the amplitude is the most-reliable parameter of VEMP tests, the amplitude needs to be corrected based on the activity of muscle contraction.2 Several techniques have been designed for monitoring muscle activity during VEMP tests. One of the reliable methods is to record the muscle activity for 20 ms before each auditory stimulus.4 Another method involves using an external monitoring device to estimate muscle action potentials during the test.13,14 While evaluating ocular VEMPs, which represent excitatory potentials originating from the inferior oblique muscle, maintaining an upward gaze increases the amplitude of the VEMPs. This gaze position also pulls the inferior oblique muscle close to the surface electrode, which can increase the recording efficacy of ocular VEMPs.15
The amplitude of VEMPs is known to decrease in the elderly.4 In addition, the tuning frequency of VEMPs—the stimulus frequency and intensity that is most effective at generating VEMPs—changes with aging.1,2 The optimal stimulus frequency for generating cervical and ocular VEMPs ranges from 750 Hz to 1 kHz in the elderly, while it is typically 500 Hz in young subjects.16 Therefore, when evaluating VEMPs in the elderly, additional testing at 1 kHz may be needed when a 500-Hz stimulus does not elicit VEMPs. The threshold intensity is also lower in the young than the elderly.10 These differences in the tuning frequency in the elderly reflect age-related changes in the intrinsic characteristics of the otolith organs.
Changes in VEMP tuning have also been reported in some pathological conditions. In an ear affected by MD, the tuning of VEMP typically shifts to a frequency of 750 Hz to 1 kHz, and higher that than in the unaffected ear.17-21 In SCD, the amplitudes of cervical and ocular VEMPs increase for both low- and high-frequency stimuli, thereby resulting in a broader frequency tuning and lower thresholds.10,22-25
In patients with acute spontaneous vertigo, horizontal and torsional spontaneous nystagmus, positive head impulse test opposite to the direction of nystagmus (presence of catch-up saccades in the direction opposite to head rotation), and the absence of other central ocular motor signs and hearing loss could indicate the presence of an acute isolated peripheral vestibulopathy, which is usually called vestibular neuritis (VN).26,27 VN is mostly diagnosed based on the clinical context, and laboratory tests can help to determine its extent. Therefore, abnormal ocular VEMPs are anticipated in patients with superior VN, while abnormal cervical VEMPs are expected in patients with inferior VN (Fig. 2). Ocular and cervical VEMPs can be abnormal when both superior and inferior nerves are involved.28-30 In atypical cases of VN in which the inflammation is scattered over the vestibular labyrinth rather than spreading through the nerve branch, VEMP tests would be useful for localizing the lesions.31
MD is a vestibular disorder characterized by the clinical triad of episodic vertigo, low-frequency sensorineural hearing loss, and tinnitus.32 Endolymphatic hydrops is a pathological hallmark of MD that develops initially in the saccule and in the apical turn of the cochlea.33 It has therefore been of interest to determine whether VEMP tests could help diagnose MD and also whether the VEMP findings differ between MD and other vestibular disorders.
Both cervical and ocular VEMPs have been reported to be abnormal in MD patients compared with normal subjects.1,2 Some studies have found that ocular and cervical VEMP abnormalities might be useful for differentiating or predicting MD in isolated auditory or vestibular syndromes such as acute low-frequency sensorineural hearing loss34 and benign recurrent vertigo without hearing loss.14 These suggestions seem reasonable given where endolymphatic hydrops initially develops.33 To enhance the sensitivity and specificity of VEMP in diagnosing MD, a parameter evaluating the tuning property of VEMPs (as mentioned above) was also introduced recently.35,36 However, all of these suggestions have limitations associated with retrospective study designs and the inclusion of populations within a narrow spectrum. Therefore, while VEMPs can be used to assess otolith function in MD, their usefulness in diagnostic testing for MD has not yet been established.
Vestibular migraine (VM) is a variant form of migraine that is one of the common disorders that results in recurrent spontaneous vertigo.37 Although its exact pathophysiology has not been established, VM is assumed to share the pathophysiology of migraine, which makes it difficult to infer the association between VM and otolith dysfunction.38 This situation has resulted in studies evaluating VEMPs in VM being rarer than those involving MD. A few studies have identified cervical or ocular VEMP abnormalities in comparisons with healthy subjects and patients with migraine without vestibular symptoms.39,40 However, VEMP abnormalities in VM have been less marked than those in MD.41,42 The abnormal VEMPs in VM could be ascribed to the concurrent existence of MD,43,44 or they may result from functional rearrangement of brainstem and cerebellum in VM. However, as with VEMP studies of MD, the limitations of these findings mean that the role of VEMPs in VM also remains to be established.
VEMP tests are not necessary for diagnosing benign paroxysmal positional vertigo (BPPV), but such tests may be useful for identifying otolith dysfunction in patients with BPPV. Indeed, BPPV can occur in association with MD or VN.45,46 Recent studies have shown that patients with BPPV commonly exhibit VEMP abnormalities that can be unilateral or bilateral.47,48 Abnormal VEMP findings have also been reported to be associated with the recurrence or the response to the repositioning maneuver.49,50
A defect in the temporal bone around the superior semicircular canal can provide a third-mobile window within the inner ear that results in a distinct vestibular syndrome.51 The typical clinical manifestations of superior canal dehiscence (SCD) are sound-/pressure-induced vertigo/nystagmus, pulsatile tinnitus, and hyperacusis. In pure-tone audiometry, the air-bone gap within a low-frequency range in addition to enhanced bone conduction inducing a negative threshold can aid the diagnosis.52 VEMPs could also provide a specific diagnostic clue for the diagnosis of SCD. Both ocular and cervical VEMPs are known to exhibit elevated responses to auditory clicks and tone bursts, with the threshold decreasing to 75 dB SPL, such that VEMPs are not inducible in the normal ear (Fig. 3).1,2 Therefore, a VEMP threshold test should be applied to patients with the clinical manifestations of SCD. Since recovery of the VEMP amplitude and threshold after surgical treatment for SCD has been reported, VEMPs can also be useful for monitoring the surgical outcomes.53
The vestibular nerve—which carries otolith information—enters the brainstem to project to the cerebral cortex, ocular motor nuclei, and spinal motor neurons.54 Central lesions disrupting the otolith pathway can therefore result in the ocular tilt reaction and subjective visual vertical tilt.55 Abnormal VEMP findings have also been reported in central lesions involving either direct or indirect pathways.56,57 Pontomedullary lesions often result in abnormal ocular and cervical VEMPs by directly disrupting the vestibular nucleus complexes or otolith pathways. The cerebellum has a reciprocal connection with the vestibular nuclei as well as primary afferent fibers from otolith organs. This can result in cerebellar lesions causing an imbalance of neural activity with respect to the otolith signals and hence abnormal VEMP findings. However, care is needed when interpreting VEMP abnormalities in central lesions since such abnormalities can vary with the characteristics, extent, and location of lesions.56,57
VEMP tests are useful for evaluating patients with vestibular symptoms. However, in most cases these tests cannot result in a definitive diagnosis, instead providing information about the severity and extent of otolithic dysfunction and facilitating the understanding of the symptoms and signs of a patient. Further well-designed investigations into the mechanisms suggested to date are warranted for increasing the usefulness of VEMP tests in clinical practice.
ACKNOWLEDGMENTS
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (2020R1A2C4002281).
REFERENCES
1. Fife TD, Colebatch JG, Kerber KA, Brantberg K, Strupp M, Lee H, et al. Practice guideline: cervical and ocular vestibular evoked myogenic potential testing: report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2017; 89:2288–2296.
2. Rosengren SM, Colebatch JG, Young AS, Govender S, Welgampola MS. Vestibular evoked myogenic potentials in practice: Methods, pitfalls and clinical applications. Clin Neurophysiol Pract. 2019; 4:47–68.

3. Colebatch JG, Halmagyi GM, Skuse NF. Myogenic potentials generated by a click-evoked vestibulocollic reflex. J Neurol Neurosurg Psychiatry. 1994; 57:190–197.

4. Welgampola MS, Colebatch JG. Vestibulocollic reflexes: normal values and the effect of age. Clin Neurophysiol. 2001; 112:1971–1979.

5. Todd NP, Rosengren SM, Aw ST, Colebatch JG. Ocular vestibular evoked myogenic potentials (OVEMPs) produced by air- and bone-conducted sound. Clin Neurophysiol. 2007; 118:381–390.

6. Rosengren SM, McAngus Todd NP, Colebatch JG. Vestibular-evoked extraocular potentials produced by stimulation with bone-conducted sound. Clin Neurophysiol. 2005; 116:1938–1948.

7. Young ED, Fernández C, Goldberg JM. Responses of squirrel monkey vestibular neurons to audio-frequency sound and head vibration. Acta Otolaryngol. 1977; 84:352–360.

8. McCue MP, Guinan JJ Jr. Spontaneous activity and frequency selectivity of acoustically responsive vestibular afferents in the cat. J Neurophysiol. 1995; 74:1563–1572.

9. Lin MY, Timmer FC, Oriel BS, Zhou G, Guinan JJ, Kujawa SG, et al. Vestibular evoked myogenic potentials (VEMP) can detect asymptomatic saccular hydrops. Laryngoscope. 2006; 116:987–992.

10. Taylor RL, Bradshaw AP, Halmagyi GM, Welgampola MS. Tuning characteristics of ocular and cervical vestibular evoked myogenic potentials in intact and dehiscent ears. Audiol Neurootol. 2012; 17:207–218.

11. Vanspauwen R, Wuyts FL, Van De Heyning PH. Validity of a new feedback method for the VEMP test. Acta Otolaryngol. 2006; 126:796–800.

12. Rosengren SM. Effects of muscle contraction on cervical vestibular evoked myogenic potentials in normal subjects. Clin Neurophysiol. 2015; 126:2198–2206.

13. Lee KJ, Kim MS, Son EJ, Lim HJ, Bang JH, Kang JG. The usefulness of rectified VEMP. Clin Exp Otorhinolaryngol. 2008; 1:143–147.

14. Lee SU, Kim HJ, Choi JY, Koo JW, Kim JS. Abnormal cervical vestibular-evoked myogenic potentials predict evolution of isolated recurrent vertigo into Meniere’s disease. Front Neurol. 2017; 8:463.

15. Rosengren SM, Colebatch JG, Straumann D, Weber KP. Why do oVEMPs become larger when you look up? Explaining the effect of gaze elevation on the ocular vestibular evoked myogenic potential. Clin Neurophysiol. 2013; 124:785–791.

16. Piker EG, Jacobson GP, Burkard RF, McCaslin DL, Hood LJ. Effects of age on the tuning of the cVEMP and oVEMP. Ear Hear. 2013; 34:e65–e73.

17. Maxwell R, Jerin C, Gürkov R. Utilisation of multi-frequency VEMPs improves diagnostic accuracy for Meniere’s disease. Eur Arch Otorhinolaryngol. 2017; 274:85–93.

18. Rauch SD, Zhou G, Kujawa SG, Guinan JJ, Herrmann BS. Vestibular evoked myogenic potentials show altered tuning in patients with Ménière’s disease. Otol Neurotol. 2004; 25:333–338.

19. Node M, Seo T, Miyamoto A, Adachi A, Hashimoto M, Sakagami M. Frequency dynamics shift of vestibular evoked myogenic potentials in patients with endolymphatic hydrops. Otol Neurotol. 2005; 26:1208–1213.

20. Winters SM, Berg IT, Grolman W, Klis SF. Ocular vestibular evoked myogenic potentials: frequency tuning to air-conducted acoustic stimuli in healthy subjects and Ménière’s disease. Audiol Neurootol. 2012; 17:12–19.

21. Sandhu JS, Low R, Rea PA, Saunders NC. Altered frequency dynamics of cervical and ocular vestibular evoked myogenic potentials in patients with Ménière’s disease. Otol Neurotol. 2012; 33:444–449.

22. Manzari L, Burgess AM, McGarvie LA, Curthoys IS. Ocular and cervical vestibular evoked myogenic potentials to 500 Hz fz bone-conducted vibration in superior semicircular canal dehiscence. Ear Hear. 2012; 33:508–520.

23. Rosengren SM, Aw ST, Halmagyi GM, Todd NP, Colebatch JG. Ocular vestibular evoked myogenic potentials in superior canal dehiscence. J Neurol Neurosurg Psychiatry. 2008; 79:559–568.

24. Govender S, Fernando T, Dennis DL, Welgampola MS, Colebatch JG. Properties of 500Hz air- and bone-conducted vestibular evoked myogenic potentials (VEMPs) in superior canal dehiscence. Clin Neurophysiol. 2016; 127:2522–2531.

25. Roditi RE, Eppsteiner RW, Sauter TB, Lee DJ. Cervical vestibular evoked myogenic potentials (cVEMPs) in patients with superior canal dehiscence syndrome (SCDS). Otolaryngol Head Neck Surg. 2009; 141:24–28.

26. Kim JS. When the room is spinning: experience of vestibular neuritis by a neurotologist. Front Neurol. 2020; 11:157.

28. Kim HA, Hong JH, Lee H, Yi HA, Lee SR, Lee SY, et al. Otolith dysfunction in vestibular neuritis: recovery pattern and a predictor of symptom recovery. Neurology. 2008; 70:449–453.

29. Shin BS, Oh SY, Kim JS, Kim TW, Seo MW, Lee H, et al. Cervical and ocular vestibular-evoked myogenic potentials in acute vestibular neuritis. Clin Neurophysiol. 2012; 123:369–375.

31. Park JY, Choi SY, Choi JH, Choi KD. Vestibular neuritis selectively involving posterior canal and utricle. J Neurol. 2018; 265:1940–1942.

32. Lopez-Escamez JA, Carey J, Chung WH, Goebel JA, Magnusson M, Mandalà M, et al. Diagnostic criteria for Ménière’s disease. J Vestib Res. 2015; 25:1–7.
33. Sperling NM, Paparella MM, Yoon TH, Zelterman D. Symptomatic versus asymptomatic endolymphatic hydrops: a histopathologic comparison. Laryngoscope. 1993; 103:277–285.
34. Wu CL, Young YH. Vestibular evoked myogenic potentials in acute low-tone sensorineural hearing loss. Laryngoscope. 2004; 114:2172–2175.

35. Singh NK, Barman A. Frequency-amplitude ratio of ocular vestibular-evoked myogenic potentials for detecting Ménière’s disease: a preliminary investigation. Ear Hear. 2016; 37:365–373.
36. Murofushi T, Tsubota M, Suizu R, Yoshimura E. Is alteration of tuning property in cervical vestibular-evoked myogenic potential specific for Ménière’s disease? Front Neurol. 2017; 8:193.

37. Lempert T, Olesen J, Furman J, Waterston J, Seemungal B, Carey J, et al. Vestibular migraine: diagnostic criteria. J Vestib Res. 2012; 22:167–172.

38. Espinosa-Sanchez JM, Lopez-Escamez JA. New insights into pathophysiology of vestibular migraine. Front Neurol. 2015; 6:12.

39. Zaleski A, Bogle J, Starling A, Zapala DA, Davis L, Wester M, et al. Vestibular evoked myogenic potentials in patients with vestibular migraine. Otol Neurotol. 2015; 36:295–302.

40. Makowiec KF, Piker EG, Jacobson GP, Ramadan NM, Roberts RA. Ocular and cervical vestibular evoked myogenic potentials in patients with vestibular migraine. Otol Neurotol. 2018; 39:e561–e567.

41. Dlugaiczyk J, Habs M, Dieterich M. Vestibular evoked myogenic potentials in vestibular migraine and Ménière’s disease: cVEMPs make the difference. J Neurol. 2020; Jun. 3. [Epub]. DOI:10.1007/s00415-020-09902-4.
42. Salviz M, Yuce T, Acar H, Taylan I, Yuceant GA, Karatas A. Diagnostic value of vestibular-evoked myogenic potentials in Ménière’s disease and vestibular migraine. J Vestib Res. 2016; 25:261–266.

43. Radtke A, Lempert T, Gresty MA, Brookes GB, Bronstein AM, Neuhauser H. Migraine and Ménière’s disease: is there a link? Neurology. 2002; 59:1700–1704.
44. Murofushi T, Tsubota M, Kitao K, Yoshimura E. Simultaneous presentation of definite vestibular migraine and definite Ménière’s disease: overlapping syndrome of two diseases. Front Neurol. 2018; 9:749.

45. Balatsouras DG, Ganelis P, Aspris A, Economou NC, Moukos A, Koukoutsis G. Benign paroxysmal positional vertigo associated with Meniere’s disease: epidemiological, pathophysiologic, clinical, and therapeutic aspects. Ann Otol Rhinol Laryngol. 2012; 121:682–688.

46. Mandalà M, Santoro GP, Awrey J, Nuti D. Vestibular neuritis: recurrence and incidence of secondary benign paroxysmal positional vertigo. Acta Otolaryngol. 2010; 130:565–567.

47. Oya R, Imai T, Takenaka Y, Sato T, Oshima K, Ohta Y, et al. Clinical significance of cervical and ocular vestibular evoked myogenic potentials in benign paroxysmal positional vertigo: a meta-analysis. Eur Arch Otorhinolaryngol. 2019; 276:3257–3265.

48. Kim EJ, Oh SY, Kim JS, Yang TH, Yang SY. Persistent otolith dysfunction even after successful repositioning in benign paroxysmal positional vertigo. J Neurol Sci. 2015; 358:287–293.

49. Lee JD, Park MK, Lee BD, Lee TK, Sung KB, Park JY. Abnormality of cervical vestibular-evoked myogenic potentials and ocular vestibular-evoked myogenic potentials in patients with recurrent benign paroxysmal postitional vertigo. Acta Otolaryngol. 2013; 133:150–153.

50. Yetiser S, Ince D, Gul M. An analysis of vestibular evoked myogenic potentials in patients with benign paroxysmal positional vertigo. Ann Otol Rhinol Laryngol. 2014; 123:686–695.

51. Minor LB, Solomon D, Zinreich JS, Zee DS. Sound- and/or pressure-induced vertigo due to bone dehiscence of the superior semicircular canal. Arch Otolaryngol Head Neck Surg. 1998; 124:249–258.

52. Ward BK, Carey JP, Minor LB. Superior canal dehiscence syndrome: lessons from the first 20 years. Front Neurol. 2017; 8:177.

53. Welgampola MS, Myrie OA, Minor LB, Carey JP. Vestibular-evoked myogenic potential thresholds normalize on plugging superior canal dehiscence. Neurology. 2008; 70:464–472.

54. Newlands SD, Vrabec JT, Purcell IM, Stewart CM, Zimmerman BE, Perachio AA. Central projections of the saccular and utricular nerves in macaques. J Comp Neurol. 2003; 466:31–47.

55. Kim HJ, Kim S, Park JH, Kim JS. Altered processing of otolithic information in isolated lateral medullary infarction. J Neurol. 2016; 263:2424–2429.

Fig. 1.
Stimulus and neural substrates of vestibular-evoked myogenic potentials (VEMPs). AC, air-conducted; BC, bone-conducted sounds; IO, inferior oblique muscle; III, oculomotor nucleus; IV, trochlear nucleus; MLF, medial longitudinal fasciculus; VI, abducens nucleus; VNc, vestibular nucleus; U, utricle; VST, vestibulospinal tract; S, saccule; SCM, sternocleidomastoid muscle.
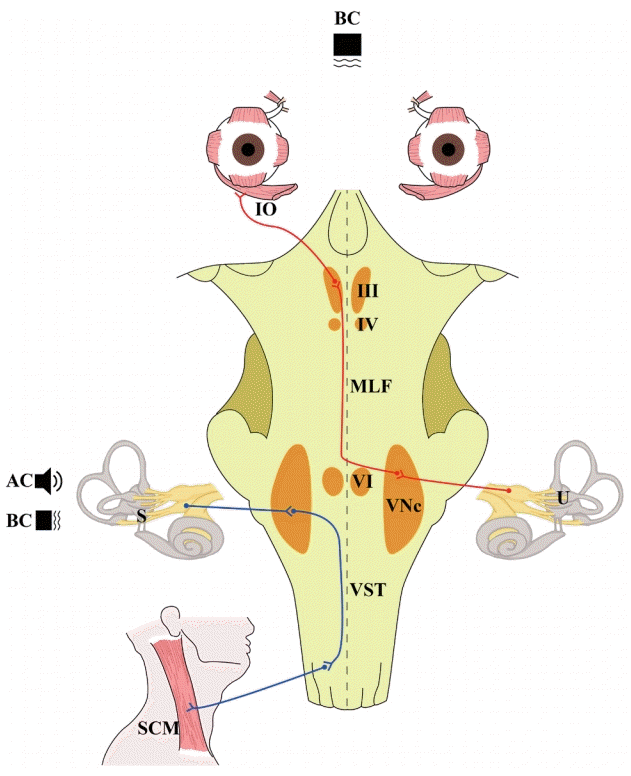
Fig. 2.
Vestibular-evoked myogenic potential (VEMP) findings in acute vestibular neuritis (VN). VN involving both the superior and inferior divisions manifests as abnormal cervical and ocular VEMPs with caloric unresponsiveness on the affected side (left column). In contrast, superior VN causes abnormal ocular VEMPs and caloric weakness (middle column), while inferior VN only decreases cervical VEMPs (right column). L, left; R, right.
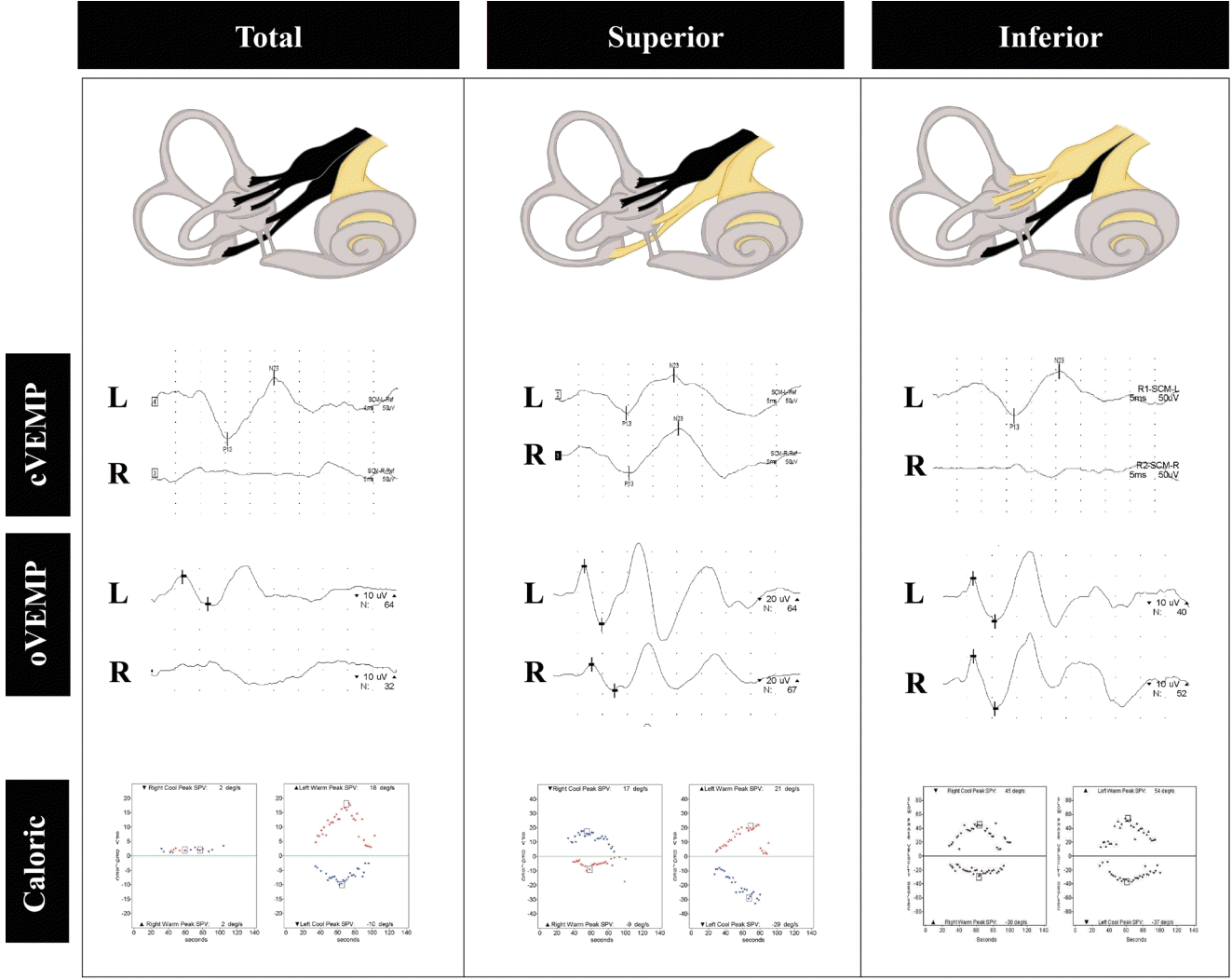
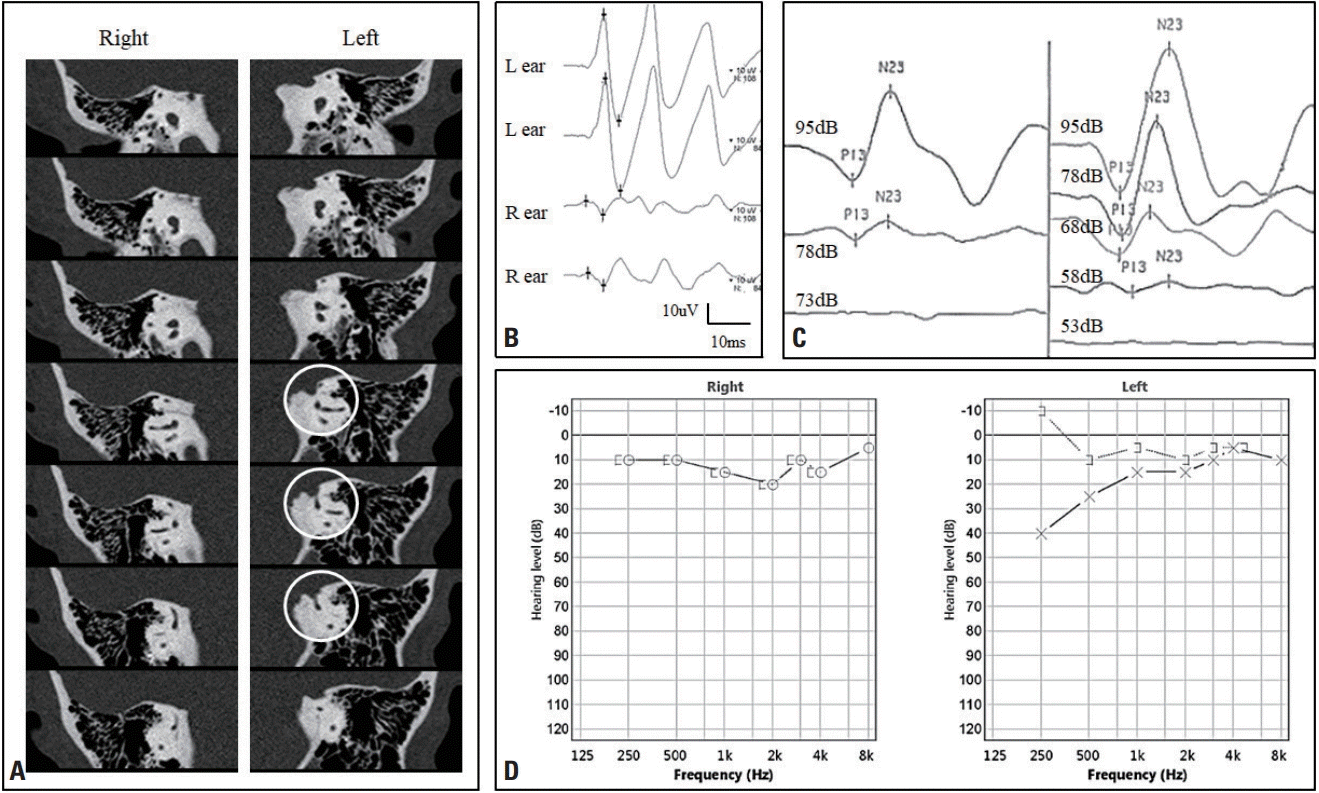
Fig. 3.
Clinical findings of superior canal dehiscence (SCD). (A) Temporal bone computed tomography showing right-side SCD (white circles). (B) Ocular vestibular-evoked myogenic potentials (VEMPs) showing increased amplitudes during left-ear stimulation with a tone burst. (C) The cervical VEMP threshold test revealed a decreased threshold in the left ear compared with the right eye. (D) Pure-tone audiometry in the left ear demonstrated an air-bone gap with a low-frequency range in addition to enhanced bone conduction inducing a negative threshold.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download