This article has been
cited by other articles in ScienceCentral.
Abstract
We performed intraoperative neurophysiological monitoring (INM) during anteromesial temporal resection (AMTR) in a patient with lesional temporal lobe epilepsy. INM revealed a sudden decrease in N20 waves in somatosensory evoked potentials (SSEPs) and poor P100 waves in visual evoked potentials (VEPs). These changes developed after applying electrocoagulation in the right mesial temporal areas. Postoperative brain magnetic resonance imaging demonstrated right thalamic and medial occipital infarctions. SSEPs and VEPs monitoring can be useful for detecting posterior cerebral artery infarction in AMTR.
Go to :

Keywords: Epilepsy, Intraoperative neurophysiological monitoring, Posterior cerebral artery infarction
Intraoperative neurophysiological monitoring (INM) is a very useful method for the early detection and intervention of reversible neurological deficits that can occur during surgery involving the neural pathways.
1 However, INM in anteromesial temporal resection (AMTR) remains underutilized. The monitoring of motor evoked potentials (MEPs) during AMTR was reported to reduce the complications of postoperative weakness due to an internal capsule infarction.
2 However, somatosensory evoked potentials (SSEPs) and visual evoked potentials (VEPs) have not been evaluated previously. We report the results of INM of SSEPs, VEPs, and MEPs in a patient with temporal lobe epilepsy (TLE) who underwent AMTR.
CASE
A 26-year-old presenting male had developed TLE seizure 10 years previously. His seizure started with an epigastric rising sensation, followed by unresponsiveness, and rarely evolving to generalization. Despite taking antiepileptic medications, focal-onset seizure with impaired awareness continued a few times per week. The findings of a neurological examination were normal. Brain magnetic resonance imaging (MRI) revealed a right medial temporal tumor at the parahippocampal gyrus (
Fig. 1A,
B). During a 7-day stay at an epilepsy monitoring unit, frequent interictal spikes and 12 automotor seizures were documented, and ictal subtraction single photon emission computerized tomography demonstrated that the seizures originated from the right mesial TLE lesion. Right AMTR along with tumor removal was decided after performing functional evaluations including 18F-FDG PET, neuropsychological tests, and the Wada test.
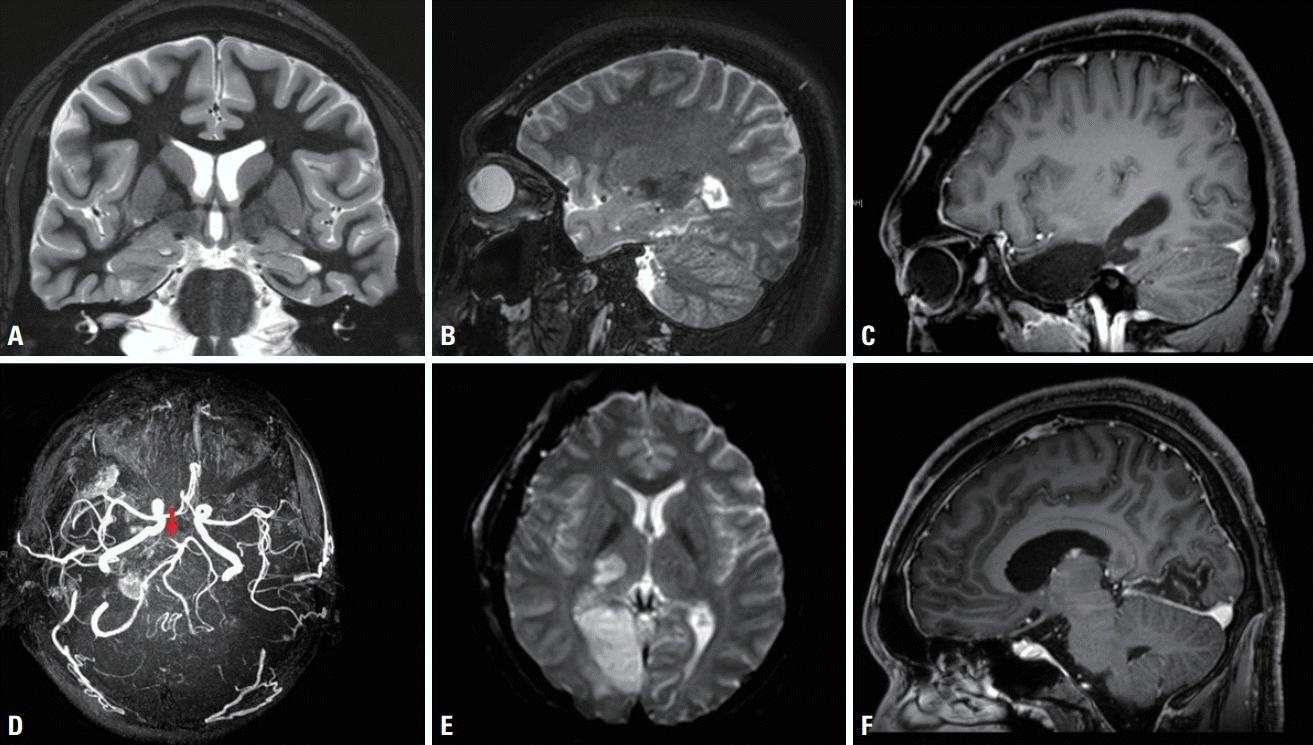 | Fig. 1.Pre- and postoperative magnetic resonance imaging (MRI) and magnetic resonance angiography (MRA) findings. (A, B) Preoperative T2-weighted oblique coronal and sagittal MRI images show an enlarged right parahippocampal gyrus with cystic changes and a malformed cortex that extend up to the tail of the hippocampus. (C) Postoperative T1-weighted sagittal MRI image shows removal of the right anteromesial temporal regions including the tumor, amygdala, and hippocampus. (D) MRA image reveals occlusion of the P1 part of the right posterior cerebral artery (PCA, arrow). (E, F) Diffusion-weighted axial and T1-weighted sagittal MRI images demonstrate ischemic lesions in the lateral part of the right thalamus as well as the mesial part of the right occipital gyrus, indicating right PCA infarction. 
|
Under total intravenous anesthesia (TIVA) induced using propofol, remifentanil, and vecuronium, multiple modalities of INM were performed during AMTR, including transcranial electrical MEPs, median and posterior tibial nerve SSEPs, and flash VEPs. For MEPs, five repetitive pulses of transhemispheric constant-voltage stimulation at 400 volts were applied at the C3 and C4 positions, which evoked polyphasic compound muscle action potentials in the contralateral arm and leg muscles (abductor pollicis brevis, abductor digiti quinti, tibialis anterior, and abductor hallucis). For SSEPs, electrical stimulation applied to the median nerve at the wrist and to the posterior tibial nerve at the ankle evoked P14 waves in the C5S area, and N20 waves at the C3’, Cz’, and C4’ electrodes when using the following parameters: 20 mA (upper extremities) or 30 mA (lower extremities), 2.9 Hz, and 50 averages. For VEPs, light-emitting diode (LED) stimulation evoked P100 waves in the left occipital (LO), middle occipital (MO), and right occipital (RO) regions according to the Queen square system.
The patient underwent right AMTR and total removal of the tumor after frontotemporal craniotomy (
Fig. 1C), during which the baseline MEPs, SSEPs, and VEPs could be reliably measured (
Fig. 2A-
C). Electrocautery was applied to bleeding that occurred during the dissection of the mesial temporal area around the tumor. After applying the electrocoagulation there was a -75% amplitude reduction of N20 waves in the SSEPs of the left median nerve, and the P100 waves became poorly formed in RO recordings of the left and right VEPs (
Fig. 3). The anesthesia level and MEPs remained stable. Interventions including increasing the blood pressure and removing a surgical retractor did not improve these changes, which persisted until monitoring was completed (
Fig. 2D-
F). The pathology was confirmed to be anaplastic epithelioid pleomorphic xanthoastrocytoma.
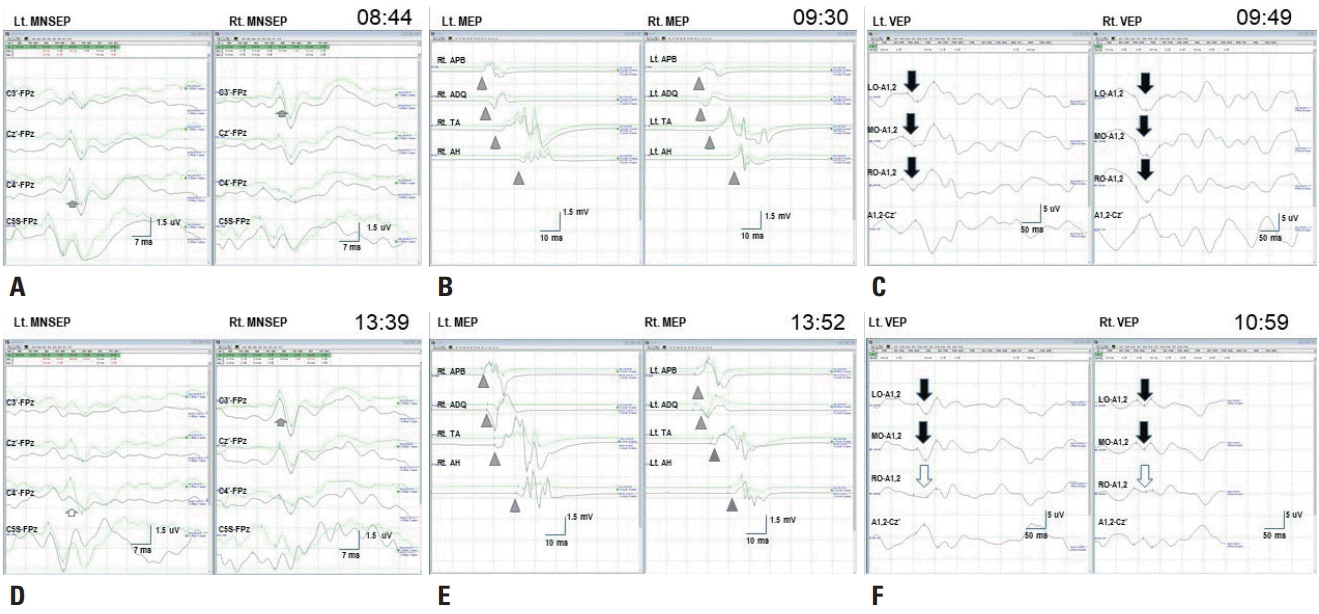 | Fig. 2.Baseline and final data recordings. (A) Baseline median nerve somatosensory evoked potential (MNSEP) show a normal N20 waves (short arrows) for each stimulation. (B) Baseline transcranial electrical motor evoked potentials (MEPs) show polyphasic compound muscle action potentials (arrowheads) at the abductor pollicis brevis, abductor digiti quinti, tibialis anterior, and abductor hallucis for stimulation on each side. (C) Baseline visual evoked potentials (VEPs) show normal P100 waves (long arrows) in the left occipital (LO), middle occipital (MO), and right occipital (RO) channels for stimulation of each eye. (D) Final data for the left MNSEP reveal decreased N20 waves (hollow short arrow) in the C4’-FPz channel but no significant change in N20 waves (short arrow) in the C3’-FPz channel for the right MNSEP. (E) Final MEPs data show no significant changes (arrowheads) in any channels. (F) Final VEPs data reveal fair wave formation (hollow long arrow) for P100 waves in the RO-A1,2 channels but no significant changes in P100 waves (long arrows) in the LO or MO recordings for stimulation of either eye. Lt., left; Rt., right. 
|
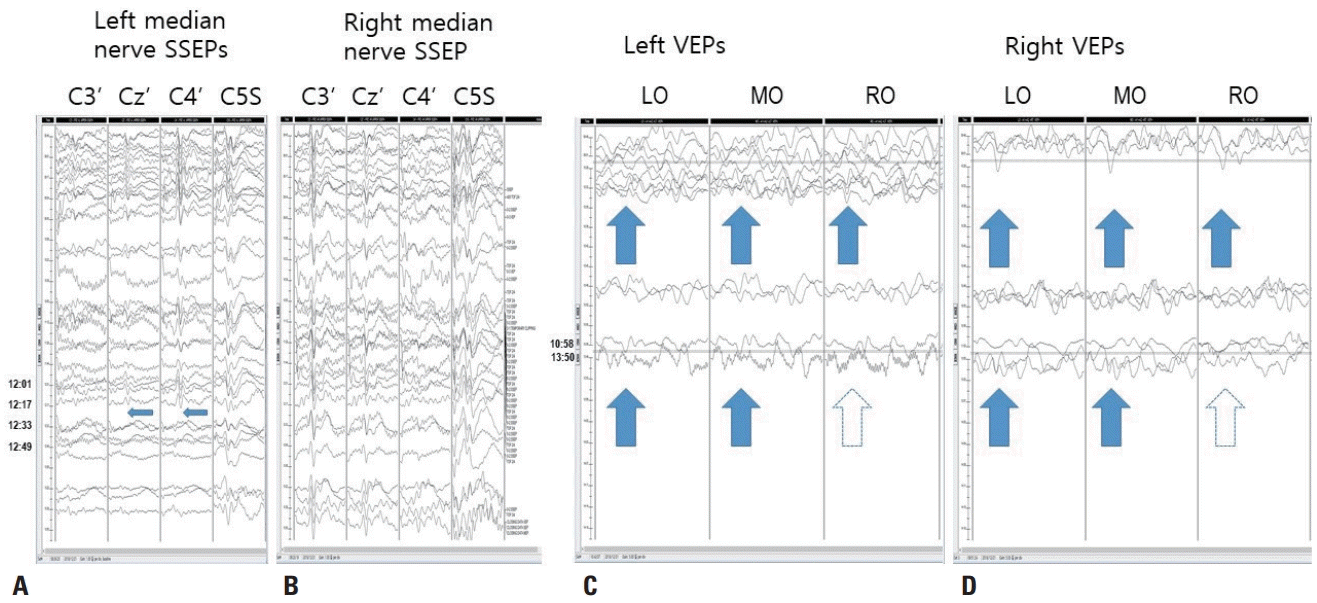 | Fig. 3.Stacked results of somatosensory evoked potentials (SSEPs) and visual evoked potentials. (A) Left median nerve SSEPs. N20 waves at Cz’ and C4’ and the P14 wave at C5S are well formed in the baseline recordings (09:48, arrows), but the N20 waves abruptly disappear between 12:17 and 12:23 (narrow arrows), while the P14 wave continues, indicating central conduction defect above the spinal level. The vertical scale is 10 mV. (B) Right median nerve SSEPs. N20 waves at C3’ and C4’ and the P14 wave at C5S are well formed. The vertical scale is 10 mV. (C, D) Left and right visual evoked potentials (VEPs). The P100 waves at left occipital (LO), middle occipital (MO), and right occipital (RO) are fairly well formed from the beginning. From 13:50, the P100 wave at LO and MO are still seen (arrows), but that at RO is poorly formed (hollow arrows). The vertical scale is 5 mV. 
|
A postoperative examination revealed left temporal upper quadrantanopia and left hemisensory deficits. Brain MRI showed two ischemic lesions in the lateral part of the right thalamus and the mesial area of the RO lobe, especially in the lingual gyrus. Brain magnetic resonance angiography (MRA) demonstrated occlusion of the P1 segment of the right posterior cerebral artery (PCA), indicating right PCA infarction (
Fig. 1D-
F).
Go to :

DISCUSSION
AMTR has been acknowledged as a very effective and safe treatment for intractable TLE, but serious neurological complications are still possible.
3 These complications can be directly related to the surgery (traction, vasospasm, edema, hemorrhage, coagulation, direct trauma, and infection) or be long-term neuropsychological effects. Even though the rate of serious complications is less than 3%, the conditions are very harmful and disabling.
4 However, INM has been rarely reported in epilepsy surgery. The decreased amplitude of N20 waves with normal P14 waves in SSEPs found in the present case suggest a lesion in the right somatosensory pathway above the cervical cord level. Considering that electrocautery was applied in the right mesial temporal areas, a new lesion in the ventroposterolateral (VPL) nucleus of the right thalamus could be suspected, since this is supplied by the thalamogeniculate artery from the P2 portion of the PCA. The lesion indicated infarction, because the SSEPs changes were strongly correlated with the regional cerebral blood flow and ischemia, predicting neurological deficits.
5,
6 Meanwhile, the poor formation of P100 waves in LO recordings of the left and right VEPs suggested the occurrence of a new retrochiasmal lesion along the visual pathway including the lateral geniculate body and occipital cortex, which are supplied by the lateral branch of the posterior choroidal artery and the PCA. Postoperative brain MRI demonstrated two ischemic lesions: 1) a right lingual gyral lesion with polar sparing, indicating a calcarine arterial infarction originating from the P4 portion of the PCA, and 2) a right lateral thalamic lesion, indicating thalamogeniculate artery infarction. Considering the location of bleeding and the use of cautery, the mechanism of the infarction could have been injury-related thromboembolism.
VEPs monitoring has recently overcome the limitations of near-field potentials in INM with the introduction of TIVA and LEDs, which has improved the reproducibility of the formation of P100 waves during aneurysmal or parasellar surgery around the anterior visual pathway.
7,
8 In the present case we monitored VEPs during AMTR of TLE around the posterior visual pathway, which detected the calcarine artery infarction. Therefore, this case provides evidence of the usefulness of VEPs monitoring for lesions in the posterior visual pathway and the anterior visual pathway.
The hippocampal region has long been considered a risky area for surgery due to its highly vascularized structure. The hippocampus is supplied mainly from the PCA via anterior, middle, and posterior hippocampal arteries, which are highly vascularized through dense connections in lateral terminal branches and anastomosis of the uncal branch of the anterior choroidal artery (AChoA) in the uncal sulcus.
9 Moreover, a tumorous lesion that is tightly attached to the surrounding structure can easily bleed during surgery. To prevent such damage, MEPs monitoring can be used to detect the AChoA suppling the internal capsule in the pyramidal pathway. SSEPs monitoring can be applied to the PCA supplying the lateral thalamus and the VPL nucleus in the somatosensory pathway, and VEPs monitoring can be applied to the posterior choroidal artery supplying the lateral geniculate body, the calcarine artery supplying the lingual gyrus, and the parieto-occipital artery supplying the cuneus. Therefore, INM for AMTR should include SSEPs, VEPs, and MEPs.
This is the first reported case of thalamic and medial occipital infarction being detected by monitoring SSEPs and VEPs during AMTR. This case supports the usefulness of SSEPs and VEPs for detecting thalamic and occipital damage during AMTR.
Go to :








 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download