Introduction
Gestational diabetes mellitus (GDM) is an important health care issue that occurs in one in six pregnant women worldwide [
1]. In the past, GDM was defined as occurring at any point in pregnancy, regardless of the extent of the disease. Recently, however, the American Diabetes Association (ADA) has clearly defined GDM as diabetes diagnosed in the second or third trimester of pregnancy [
2]. According to data from the Korean Diabetes Association in 2013, the prevalence of GDM in Korea increased from 4.1% in 2007 to 10.5% in 2011 [
3], and the incidence of GDM relative to the number of babies born in 2017 was estimated to be 15.3% [
4,
5].
The risk factors for GDM include heredity (family history) and environmental factors (age, obesity, high-fat diet, etc.) [
6], and the prevalence of GDM is expected to further increase gradually due to the increasing age of childbirth and changes towards westernized eating habits among Korean women. GDM may recur in 33% to 50% of subsequent pregnancies [
7], and GDM is associated with a high risk of type 2 diabetes, a lifelong condition [
8]. Above all, GDM has a serious impact on the mother and fetus [
2], including elevated risks of premature birth and maternal overweight, preeclampsia, cesarean section, fetal macrosomia, and trauma during delivery. After delivery, the child also has increased risks of disability due to obstetric complications, hypoglycemia, hypocalcemia, hyperbilirubinemia, respiratory distress syndrome, and obesity [
8].
Early detection is important to improve the prognosis of GDM and to reduce the risk of health-related problems in the mother and fetus, and careful health management for pregnant women is required to maintain appropriate blood glucose levels [
8,
9]. In particular, in GDM, blood glucose levels can be managed only by diet and lifestyle modifications [
1]; therefore, for management of blood glucose in pregnant women to be successful, patients themselves must have a solid knowledge of the disease and perform self-care. Consequently, health education and training are important for continuous blood glucose management [
10]. However, pregnant women who are found to have GDM may not know much about the disease or blood glucose management because they have not experienced the disease before [
10], and most pregnant women experience anxiety, depression, fear, and stress about responding to health problems that may negatively affect the fetus [
11]. The adaptations required to manage GDM, in addition to the physiological and psychological changes of pregnancy, cause additional stress regarding blood glucose control and disease burden, which can reduce the effectiveness of treatment [
12]. GDM may also adversely affect the health care behaviors of pregnant women and the formation of maternal identity through complex factors [
10]. The care goals for women with GDM are aimed at preventing complications in the mother and fetus based on early detection and treatment, with the ultimate objective of safe birth [
10]. Therefore, the control of blood glucose levels in women with GDM is paramount, and health care providers need to provide comprehensive health care interventions tailored to both the physiological changes and the individual needs of pregnant women [
9-
11]. For clinicians caring for Korean women with GDM, it would thus be beneficial to conduct a systematic analysis of health care programs (education, intervention methods, etc.) implemented for Korean women with GDM, with an analysis of their effectiveness, methods, and content. However, no such study has yet been carried out, despite the steady increase of GDM in Korea.
Purpose of research
The purpose of this study was to systematically review studies of health care programs conducted among Korean women with GDM by examining the general characteristics of the selected studies and analyzing the effectiveness of the health care programs described therein.
Go to :

Discussion
This systematic review examined the effects of seven studies that described health care programs provided to Korean pregnant women with GDM and reported the physiological, behavioral, cognitive, and psychosocial effects of those programs.
All of the studies were non-randomized experimental studies, and the majority (n=6) were carried out since 2013. More broadly, intervention studies including medication, diet, and exercise therapy have been carried out for women with GDM from diverse linguistic and cultural backgrounds [
23,
24]. The growing prevalence of GDM in Korea, related to the aging of pregnant women, seems to have contributed to an increasing interest in GDM [
20]. In the studies analyzed herein, the health care programs tended to be provided on an individual basis (n=4), rather than in small groups, and through offline delivery (n=4), although three studies used online [
19] or combined online and offline modalities [
17,
20]. Although a prior study [
11] suggested that the effects of programs might differ according to the delivery method, this study could not clearly identify any such effects due to an insufficient number of studies. Various intervention strategies and methods, including case management and information technology-based programs, were used in the research analyzed herein, and five of the seven studies included physical and lifestyle interventions, such as self-measurement of blood glucose levels, diet, and exercise. This is thought to reflect the importance of diet and exercise as ways to improve blood glucose levels in patients with GDM; exercise and diet are major management methods for GDM, just as they are for type 1 and type 2 diabetes [
20], especially since insulin alone does not provide sufficient blood glucose control in GDM. Although the effect size of the intervention method could not be determined due to the heterogeneity of the studies, researchers should investigate interventions and approaches that reflect the needs of women with GDM, considering that GDM, which is diagnosed at 24-28 weeks of pregnancy, requires regular self-care, both during pregnancy and after childbirth [
11].
Most of the selected studies (n=6) confirmed blood glucose control as a physiological outcome. The reported parameters included HbA1c, FBS, OGTT, post prandial blood sugar, and glycated albumin, and two or more physiological indicators were analyzed in four studies. The ADA and the World Health Organization recommend monitoring HbA1c, as it reflects the average blood glucose level within 3 months and serves both as a diagnostic criterion for diabetes and as an indicator of blood glucose control [
25,
26]. Although HbA1c is generally a reliable indicator, it may be affected by the physiological diabetogenic effects of pregnancy, so appropriate blood sugar testing needs to be performed starting on the first prenatal visit when GDM risk is suspected [
1]. Glycated albumin, which reflects changes in blood sugar within weeks due to the shorter half-life of albumin relative to hemoglobin, has the advantage of detecting changes in blood glucose control over relatively short intervals compared to glycated hemoglobin; in particular, it sensitively reflects post prandial blood glucose [
26]. GDM requires more stringent blood glucose control goals than type 1 or 2 diabetes [
27], since macrosomia, the main complication of GDM, is primarily related to post prandial hyperglycemia [
28]. Despite rigorous attempts to control blood glucose levels based on glycated albumin measurements [
26] and the lack of evidence that one test method is superior to the other [
29], the complications of diabetes may progress during pregnancy. One study analyzed herein focused on the postpartum period. GDM pregnancies are considered high-risk, and women with GDM are also at an elevated likelihood of developing diabetes in the future, which underscores the importance of regular blood glucose tests to prevent diabetes after delivery [
29]. Although no consensus has been reached yet on when and how to detect postpartum abnormalities in women diagnosed with GDM [
29], the ADA recommends a 75-g OGTT at 4 to 12 weeks after delivery, and every 1 to 2 years afterward [
1,
2]. As such, thorough postpartum care, including blood glucose management, is important for women with GDM [
2,
8].
There were only two studies each that reported self-care and self-management as behavioral outcome variables. An integrated self-care program, a comprehensive lifestyle-modification coaching program, and a web-based self-care program were effective for blood sugar control for among pregnant women with GDM. Since improvements in diet and exercise play a more foundational role in treating GDM than is the case for other types of diabetes, it is important to promote self-care to encourage women to actively seek out lifestyle modifications [
18]. However, many women with GDM have reported that self-care in terms of changing diet and exercise was difficult [
17]. Considering that a lack of lifestyle improvements after childbirth often leads to type 2 diabetes [
1,
16], developing health care programs that can encourage sustained self-care in terms of lifestyle improvement is important.
The single study that reported a cognitive outcome variable did not find improvement in GDM knowledge. This is possibly related to the fact that the program focused on coaching to improve self-care capability for blood glucose control rather than education. Pregnant women with GDM, in particular, have been reported to have low levels of knowledge regarding weight management, hypoglycemia treatment, and exercise methods for blood glucose control [
10]. Although improvement of knowledge does not always lead to positive behavioral changes, it is necessary to consider strategies that can improve specific knowledge when developing programs to promote self-care targeting lifestyle modifications.
While the psychosocial effects of the programs varied, including depression (n=3), anxiety (n-2), self-efficacy (n=2), and maternal identity (n=1), the number of studies was limited, making it difficult to present quantitative estimates of intervention effects. As psychosocial difficulties can have a negative effect on blood glucose management by reducing the treatment effect [
12], assessing psychosocial outcomes is important for pregnant women with GDM. Pregnant women with GDM have been reported to experience greater psychological anxiety and depression due to higher physical and psychological fatigue than their healthy counterparts [
30], concerns about maternal and fetal effects of GDM [
10], and guilt [
11]. Thus, pregnant women with GDM not only have educational needs for blood glucose management but also require emotional support to reduce the anxiety and stress they feel [
10,
11]. However, current care for GDM mainly tends to focus on checking fetal health and the physical and hormonal changes in pregnant women, often overlooking the psychological care needs of women with GDM [
16]. This review supports the need for more psychosocial interventions to promote acceptance, coping, and adaptation to GDM.
Family members or spouses did not participate in any of the interventions. However, family support as perceived by the pregnant women with GDM was important, and active support from family members was a factor associated with success for diet changes and self-care behavior in pregnant women [
11]. If the family neglects GDM or blames it on the pregnant woman, the pregnant woman may feel guilt and stress, subsequently becoming less motivated to manage her health [
12]. As the diagnosis of GDM may cause a sense of being overwhelmed, increasing women’s knowledge of GDM and ensuring cooperation in managing their health can enable pregnant women with GDM to recognize their current situation in a more positive light and to maintain stable diabetes management [
12,
30]. It would be beneficial for future interventions to engage family members as well [
11].
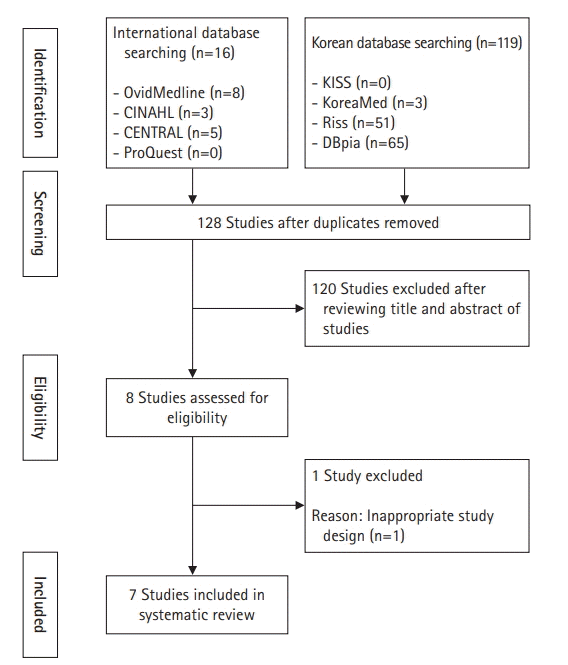
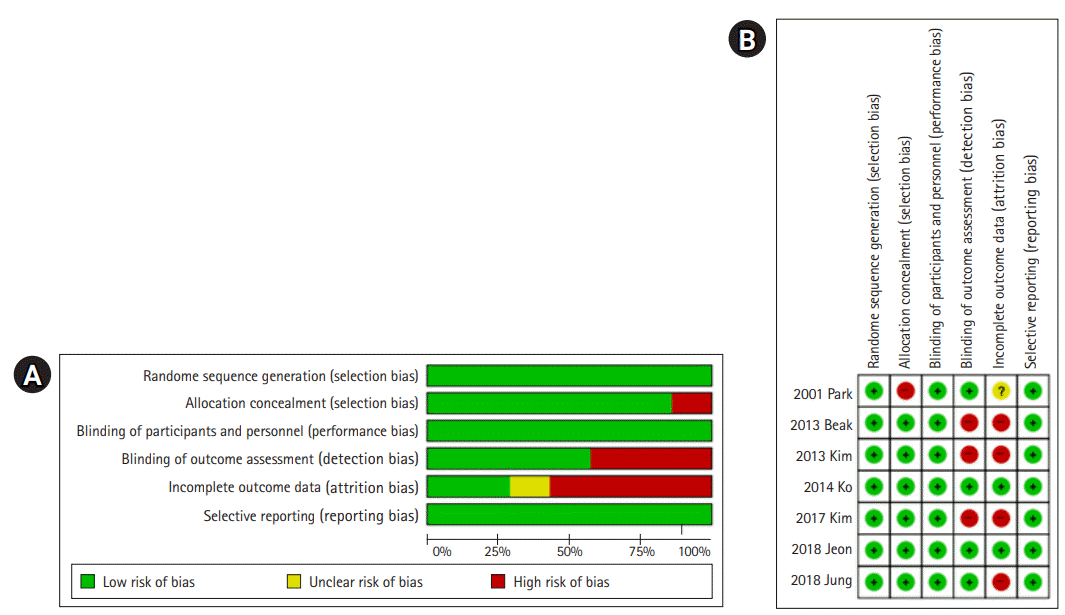
The quality of the studies included in this study was assessed as high, considering the overall low risk of bias. The number of selected studies, however, was small and all of them were non-randomized control experiment studies. Given the lack of randomized studies, the scope for generalizing and interpreting the mediating effects presented by the selected studies is limited.
Nonetheless, this review contributes to the body of knowledge on GDM by reviewing and presenting the effects of health care programs, identifying the current situation of interventional research conducted to date, and confirming the methods, content, and effects of interventions. Future studies should attempt to use a randomized controlled trial design, and meta-analyses should be conducted to clarify the clinical effects. Individual education should also be provided to identify and implement mental health-related programs that reduce negative emotions and stress, such as anxiety and depression, and the development of programs with family-oriented approaches and a focus on the educational needs for health care for pregnant women with GDM should be prioritized.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download