The gastrointestinal tract is the most common site of extra-nodal involvement in lymphoma. The stomach is the most frequent site of gastrointestinal involvement, followed by the small intestine and ileocecal area.1 Among the gastric lymphomas, primary gastric lymphoma (PGL) originates in the stomach, with or without perigastric and/or abdominal lymph node involvement. PGL is an uncommon tumor, accounting for less than 15% of gastric malignancies and about 2% of all lymphomas,1–3 and diffuse large B-cell lymphoma (DLBCL) is the most common type of primary gastric lymphoma.1 Due to the ambiguity and diversity of endoscopic findings, gastric lymphoma cannot be easily distinguished from gastric adenocarcinoma.
Although several recent studies have addressed the clinical and endoscopic features of gastric lymphoma, the diagnosis of gastric lymphoma remains a challenge, especially in the early stage of the disease, which decreases the likelihood of successful management.4,5 As it is difficult to distinguish gastric cancer from gastric lymphoma, it is also difficult to distinguish primary gastric lymphoma from gastric involvement of systemic lymphoma.
This study investigated the possible strategies for endoscopists to diagnose primary lymphoma more efficiently by addressing the diagnostic challenges. Therefore, the aim of this study was to investigate the difference in clinical and endoscopic features between primary gastric lymphoma and gastric involvement of lymphoma. We will describe three pair of cases which was difficult to tell which one is primary or secondary lymphoma case.
MATERIALS AND METHODS
Patient population
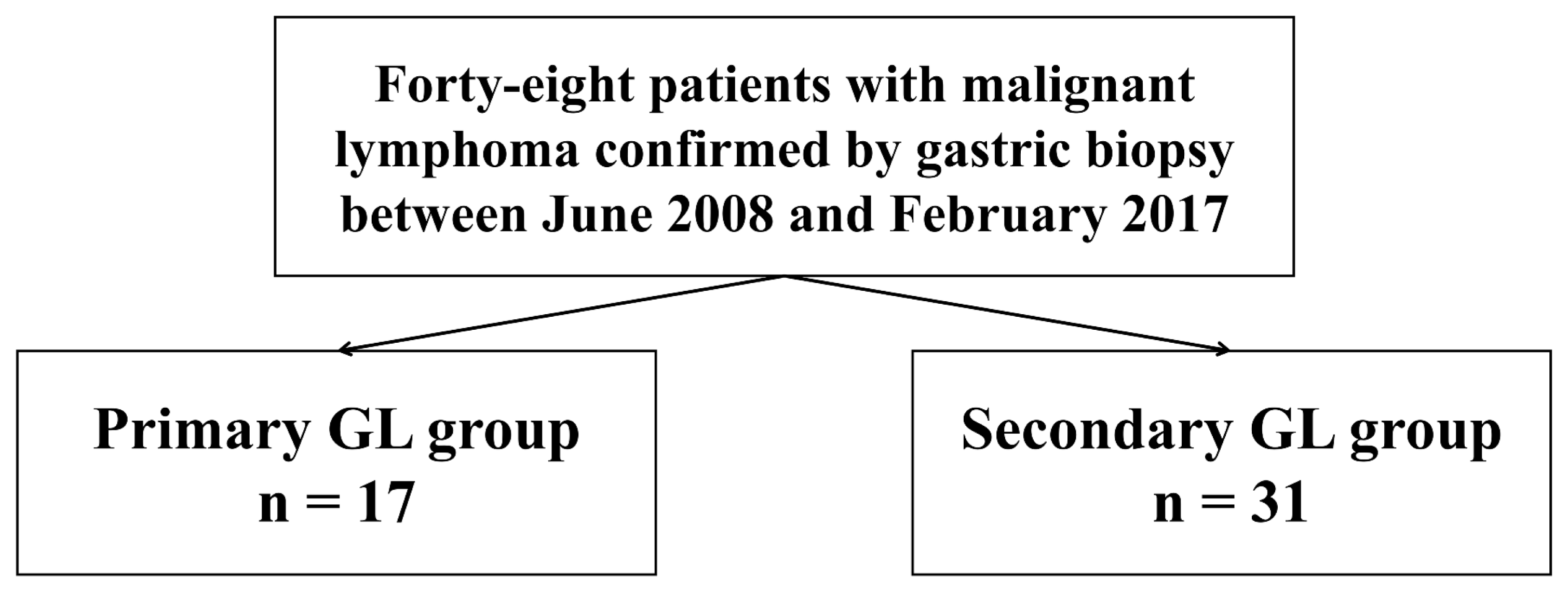
This study was a retrospective study conducted at the single tertiary center and involved lymphomas confirmed by endoscopic biopsy from June 2008 to February 2017. Forty-eight patients were enrolled in this study. The enrolled patients were divided into a primary gastric lymphoma group (primary group, n = 17) and a gastric involvement group (secondary group, n = 31) based on the presence of only gastric lesion (Fig. 1). Patients’ clinical characteristics, endoscopic findings and pathologic data were retrospectively reviewed using electronic medical records. The study was approved by our Institutional Review Board. All procedures were in accordance with the ethical standards of the Declaration of Helsinki. (IRB No.: KUGH 2019-10-021)
Primary and secondary gastric lymphoma
Primary gastric lymphoma was defined by the gastric location of the main lesion, with or without perigastric and/or abdominal lymph node involvement. 6 In the present study, the primary gastric lymphoma was defined based on the following Dawson’s criteria: 1) absence of peripheral lymphadenopathy at the time of presentation, 2) lack of enlarged mediastinal lymph nodes, 3) normal total and differential white blood cell counts, 4) predominance of gastric lesions at the time of laparotomy with only lymph nodes apparently affected in the immediate vicinity, 5) no lymphomatous involvement of liver and spleen.7 On the other hand, cases of secondary lymphoma were defined by distant lymph node involvement or involvement of other organs, deviating from the definition of primary lymphoma.
At time of initial diagnosis of gastric lymphoma, biopsy specimens from stomach, lymph node, or bone marrow were processed routinely with preparation of hematoxylin-eosin–stained sections. If initial hematoxylin-eosin stained slide showed suspicious lymphoma, fixed, paraffin-embedded tissue specimens were assessed immunohistochemically by pathologists using antibodies specific for CD3, CD5, CD10, CD20, cyclin D1, bcl-2, bcl-6, Cytokeratin cocktail, EMA and MUM-1. Some specimens also assessed for B-cell monoclonality using Immunoglobulin Heavy Chain (Ig H) gene rearrangement.
Endoscopic classification
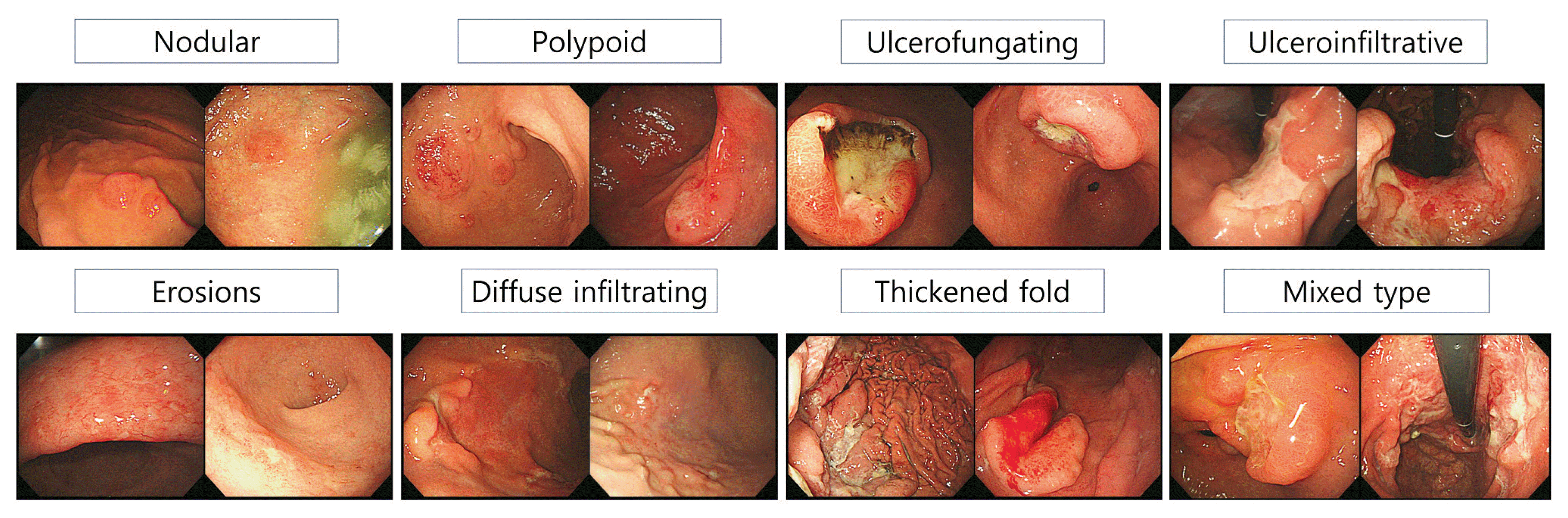
Endoscopic findings listed the most frequently mentioned items in the literature previously associated with gastric lymphoma. The most redundant and frequently mentioned findings were classified into 8 groups as follows: nodular, polypoid, ulcerofungating, ulceroinfiltrative, erosive, diffuse infiltrating, thickened fold-like, and mixed types.4,8–10 Representative cases under each classification are illustrated in Fig. 2.
Statistical analysis
The categorical variables were compared using Pearson’s chi-square, Fischer’s Exact test or linear by linear association, whereas the continuous variables were compared using Student’s t-test where appropriate. Statistical analysis was performed using IBM SPSS Statistics version 24.0 (IBM Co., Armonk, NY, USA) with a statistical significance level set at P < 0.05.
Go to : 
RESULTS
1. Baseline characteristics
The mean age of the patients was 63.3 ± 13.1 years and 29 patients were female (60.4%). There was no clinical difference between patients with primary and secondary lymphomas, except for the involvement of the fundus (P = 0.035), when the invasion site of the gastric lesion was divided into the antrum, body, and fundus, respectively (Table 1). Only 41.7% (20/48) cases considered lymphoma in the first endoscopic diagnosis, including both primary and secondary lymphoma.
Table 1
Baseline and endoscopic characteristics of patients diagnosed with primary and secondary gastric lymphoma
2. Clinicopathologic findings
DLBLC was the most frequently diagnosed pathology (81.3%) based on all the histological findings in this study, similarly to previous studies. Approximately 56% of the invasion depth in the stomach was confined to the mucosa and submucosal layer, and there was no difference in primary and secondary lesions (Table 2). Involvement of lymph node in the upper gastrointestinal tract was significantly higher in secondary than in 29.4% of primary and 90.3% of secondary cases. Bone marrow test was performed in about 90% of cases, whereas the invasion was more frequent in secondary cases than in primary lymphoma. In 43% of cases tested for Helicobacter pylori, no difference in the pathology of primary and secondary lymphomas was detected.
Table 2
Clinical and pathologic characteristics of patients according to the primary and secondary gastric lymphoma
3. Endoscopic findings
No statistically significant differences were detected in the eight different types of primary and secondary gastric lymphoma based on endoscopic findings. However, the ulceroinfiltrative type, similar to a Borrmann type 3 stomach cancer was the most common, followed by the mixed type in both groups (Table 3).
Table 3
Endoscopic findings of gastric lymphomas
4. Primary and secondary lymphoma cases difficult to distinguish
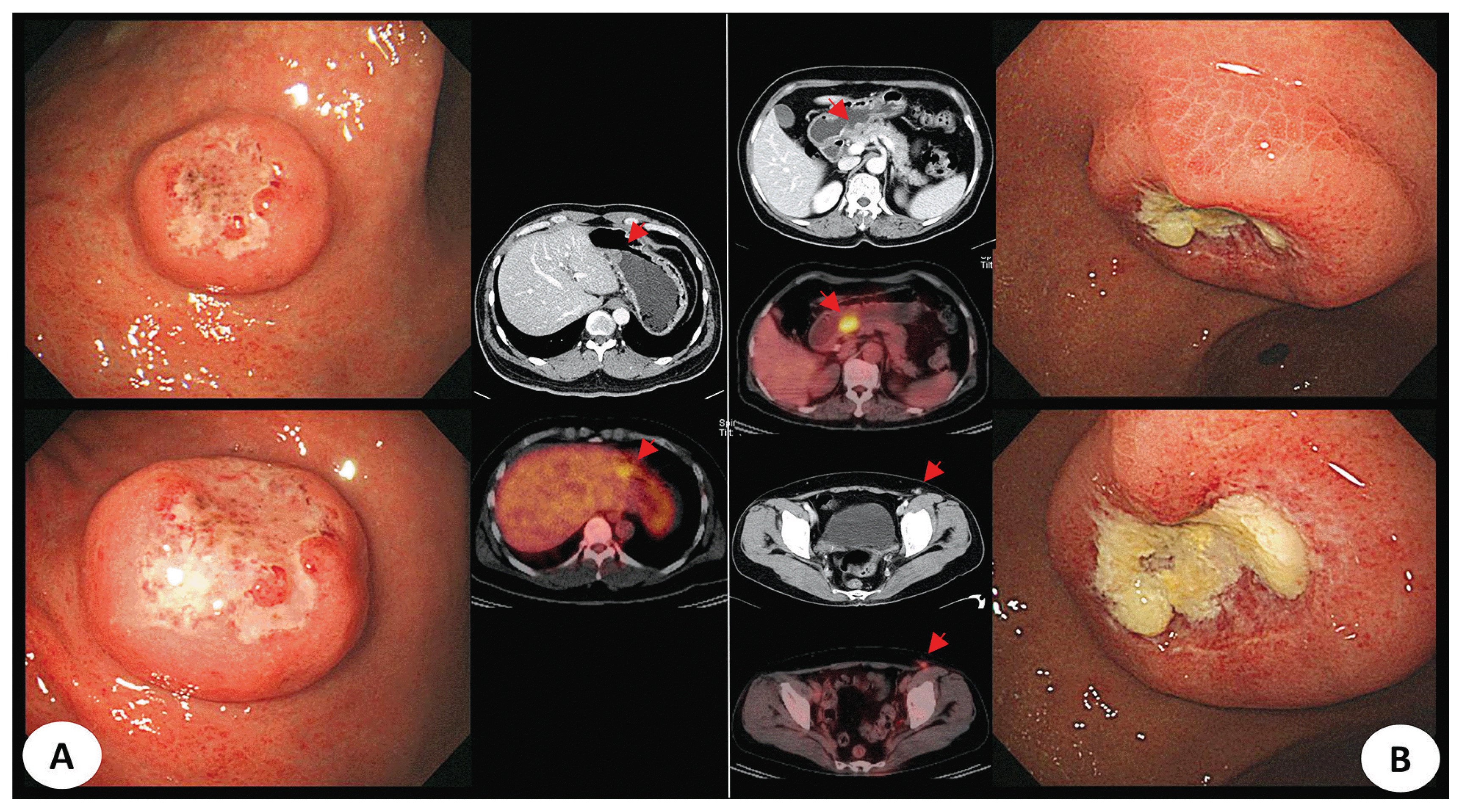
The three representative cases included in this study are as follows. First, both cases were classified as polyoid lesions, with histological findings of diffuse large B-cell lymphoma. However, a case was classified as secondary lymphoma with ileal lymph node involvement. Both cases were difficult to differentiate because of similar endoscopic features (Fig. 3). Second, both cases showed ulcercofiltrative lesion, although primary gastric lymphoma was confirmed as mantle-cell lymphoma and secondary lymphoma showed diffuse large B-cell lymphoma histology. In secondary gastric lymphoma, positron emission tomography-computed tomography (PET-CT) showed invasion of multiple whole bones, bone marrow, left orbit, liver, both adrenal glands, and prostate as well as right subclavian lymph node (Fig. 4). In the last example, two cases were classified under a mixed type endoscopically. All cases were confirmed as diffuse large B-cell lymphoma. In the latter case, lymph node involvement of the whole body including cervical, left mediastinal, both axillary area, aortocaval and both inguinal areas was confirmed in PET-CT (Fig. 5).
 | Fig. 3PET-CT features of endoscopically polypoid mass-like lesions in primary DLBCL (A) and secondary DLBCL (B) cases (Abbreviation. PET-CT, positron emission tomography-computed tomography; DLBCL, diffuse large B-cell lymphoma). |
 | Fig. 4Endoscopically ulceroinfiltrative lesions of two cases. (A) Primary gastric lymphoma which was diagnosed as mantle-cell lymphoma. (B) Secondary gastric lymphoma which was diagnosed as DLBLC. (C) Multiple involved lymph nodes and organs were observed on PET-CT in same patients of B (Abbreviation. DLBCL, diffuse large B-cell lymphoma; PET-CT, positron emission tomography-computed tomography). |
 | Fig. 5Two cases of mixed type with two or more endoscopic findings. (A) Primary DLBLC. (B) Secondary DLBLC. (C) Hypermetabolism of stomach and multiple distant lymph node was seen on PET-CT in cases of B (Abbreviation. DLBCL, diffuse large B-cell lymphoma; PET-CT, positron emission tomography-computed tomography). |
Go to : 
DISCUSSION
In the present study, we identified the clinical differences between primary and secondary lymphomas and the differences in endoscopic morphology associated with malignant gastric lymphoma, except mucosa-associated lymphoid tissue (MALT) lymphoma. Although the clinical features and endoscopic findings of gastric lymphoma were reported, no study reported the comparison of primary and secondary gastric lymphomas. We found no differences in age and gender between primary and secondary malignant gastric lymphomas. However, cases with fundus involvement were significantly higher in secondary gastric lymphoma. Similar to our study, Kolve et al showed that primary and secondary gastric NHL differed endoscopically, and the fundus was a low-risk factor for primary gastric NHL whereas unifocal growth pattern increased the risk of primary gastric lymphoma.10
DLBCL was the most common pathology underlying primary and secondary lymphoma, without any difference in invasion depth, bone marrow involvement, or Helicobacter pylori positivity between the two groups. On the other hand, regional lymph node involvement was significantly higher in secondary lymphoma. Based on endoscopic findings, ulceroinfiltrative type was the most common in both groups, and based on first impression of endoscopists, gastric lymphomas were found only in about 40%. According to a report by Cui et al.11 in a total of 168 patients suspected with endoscopic lymphoma, 35 were diagnosed with gastric lymphoma, with a diagnostic sensitivity of 20.8%, and included 54.3% ulcerative cases. Biopsy revealed DLBCL in 65.8% of the cases. On the other hand, 52.6% of the negative group showed infiltrative type, and histopathological examination showed mostly chronic inflammation (63%).
The first report of gastric lymphoma was classified as mass-forming, diffuse infiltration, with a superficial spread, and considered as unclassified type by Palmer ED8 in 1950. However, the classification was limited by lack of high-resolution endoscopy at the time. A total of 66 patients were analyzed based on endoscopic findings suggested by Palmer’s classification. Of these, 44 patients showed infiltrative findings and 24 patients had exophytic type. The most common endoscopic findings were infiltrative findings.9 Endoscopic classification was performed to distinguish polypoid, ulcerative, ulcerative-infiltrative, diffuse-infiltrating, and normal findings in a subsequent study by Kolve M et al.10 Another study by Andriani A et al.4, identified endoscopic findings involving ulcerative, exophytic, hypertrophic, mucosal petechial hemorrhage, and normal mucosa types. However, this study excluded MALT lymphoma, a type of low-grade lymphoma, and thus did not appear to be a normal mucosa in the endoscopic classification. This normal finding was not included in this study.
Although endoscopic studies of previous gastric lymphoma are few, they reveal the following features. Ghimire P et al.1 concluded that endoscopic evaluation failed to distinguish AGC and gastric lymphoma, although ulceration, diffuse infiltration, and polypoid mass were non-specific but representative findings. Ge et al.12 analyzed 87 patients with gastrointestinal lymphoma including 46 gastric cases and found that antrum (43.5%) was the most involved. However, more than half of the patients included in this study were MALT patients, suggesting a difference in prognosis between malignant gastric lymphoma and early MALT lymphoma. In study of Zeggai et al.13 lymphoma was divided into high and low grades in the Western world. However, ulcerative lesions were the most common macroscopic features regardless of grade, gastric or intestinal lesions. In a case report, Li and Jiang14 reported hyperemic changes or glass-like submucosal lesions in mantle-cell lymphoma. In our study, ulceroinfiltrative type was the most common, and erosion and polypoid types were similar to those reported in previous studies.
This study has several limitations. First, this study included only 48 patients with malignant lymphoma, excluding MALT lymphoma, in gastric biopsy. Therefore, as the number of patients increases, additional differences in primary and secondary cases can be expected. Second, this study is a retrospective analysis. Therefore, investigations such as Helicobacter pylori test were not performed. However, because it is a rare disease, it may be difficult to conduct prospective analysis. Finally, based on the gastric lymphoma progression, cases involving the initial diagnosis of primary gastric lymphoma may be classified as secondary gastric lymphoma in the future. However, because of the limited number of studies investigating endoscopic findings of gastric lymphoma, our analysis represents a meaningful approach.
In conclusion, malignant gastric lymphoma is not easy to distinguish from advanced gastric cancer. Moreover, endoscopic differentiation of primary and secondary gastric lymphoma is difficult. However, when the lesion is present in the fundus, we keep in mind the possibility of secondary gastric lymphoma.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download