Abstract
Although stroke is common in infective endocarditis (IE), only 26 cases of thrombectomy have been reported to date for IE-related acute stroke. We report a 40-year-old man who presented with left middle cerebral artery occlusion of unknown cause. Multiple attempts of mechanical aspiration thrombectomy and stentrievers failed to recanalize the artery. Effective revascularization was eventually achieved by placing a self-expanding intracranial stent. Post-procedurally the patient was diagnosed with IE with mitral valve insufficiency, mandating emergent valvular replacement while the patient was still on tirofiban infusion. On follow-up, the patient had a modified Rankin’s score of 0, had no recurrent stroke, and the intracranial stent remained patent yet stenosed. Based on the use of a self-expanding intracranial stent in the setting of IE, we discuss the consequences of the fibrotic and inflammatory content of the embolus and the associated high risk of intracranial hemorrhage which complicates clinical decision making.
Go to : 
The initial treatment for infective endocarditis (IE) is antibiotic therapy to reduce mortality and morbidity related to heart failure and systemic embolism. Stroke occurs in about 20% of all IE cases9) and may result in a mortality rate of up to 56 percent.8) Intravenous or intraarterial thrombolysis for IE-related stroke is not well-defined in the literature.8)10) Notwithstanding the few successful cases of systemic thrombolytic therapy reported in the literature,6) an increased risk of intracerebral hemorrhage has repeatedly been documented.3) In addition, the overall thrombolysis results are not encouraging.13) Some authors claimed that intravenous thrombolysis is contraindicated in acute stroke secondary to infective Endocarditis.1) There are 26 cases of mechanical thrombectomy for IE-related acute stroke in the literature.1)2)5)8–12) We describe periprocedural and postprocedural management and clinical challenges of a case of acute IE associated with embolic stroke.
Go to : 
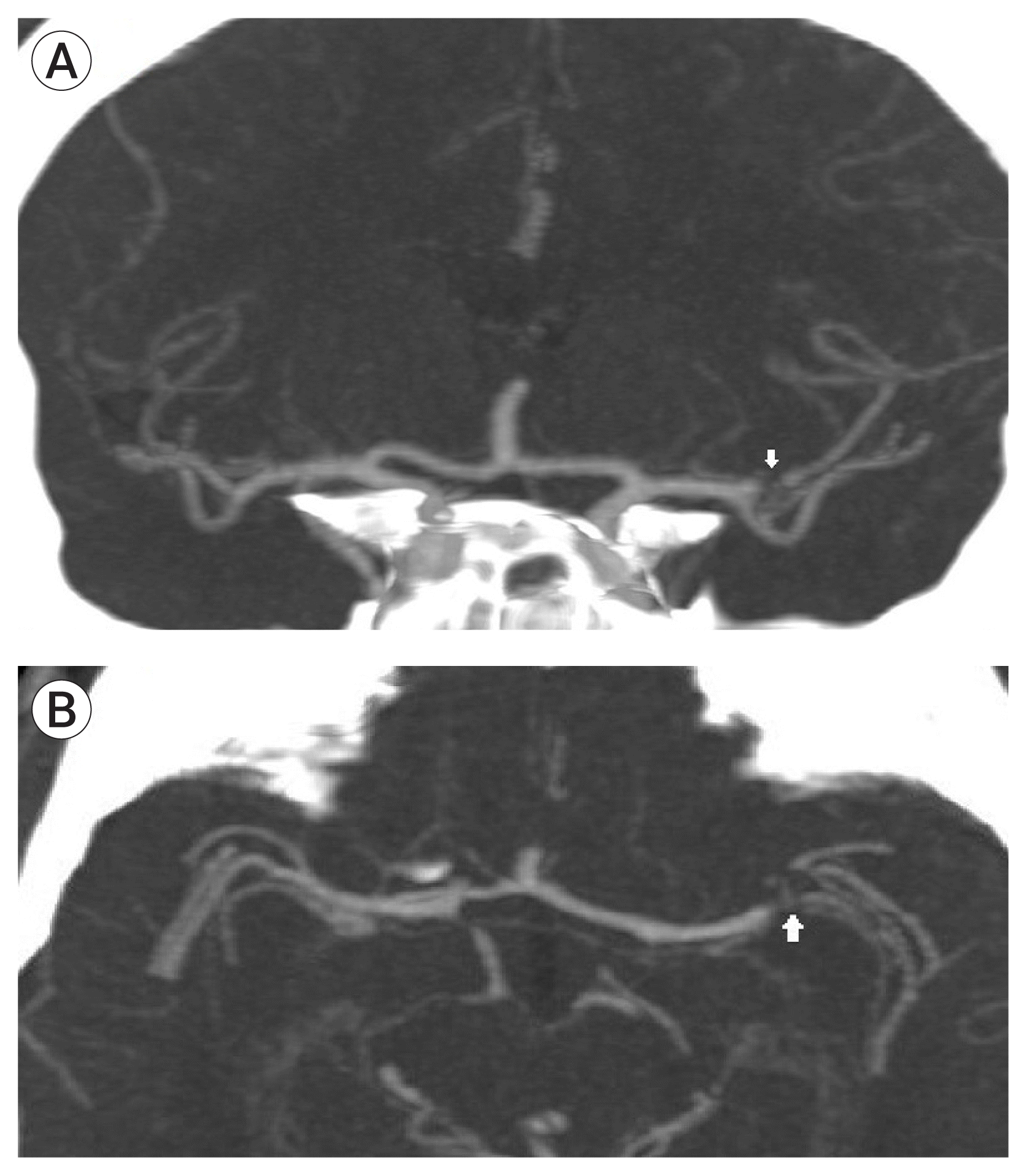
A 40-year-old male presented to our emergency department with an acute onset of dysarthria and facial paralysis. He had been investigated for fewer of unknown origin for the last several weeks. At the time of admission, his temperature was 36°C, blood pressure was 140/80 mm Hg, respiratory rate was 18, his pulse was regular (107 bpm), and the Jump to search the National Institutes of Health Stroke Scale (NIHSS) score was 3. Initial imaging work-up revealed no signs of ischemia on a non-contrast head computed tomography (CT) and occlusion of the superior trunk of the left middle cerebral artery on CT-angiography (Fig. 1). Three hours after the onset of stroke, IV tissue plasminogen activator (t-PA) was initiated. Despite the presence of low NIHSS score, he was transferred to the angiography suite for revascularization.
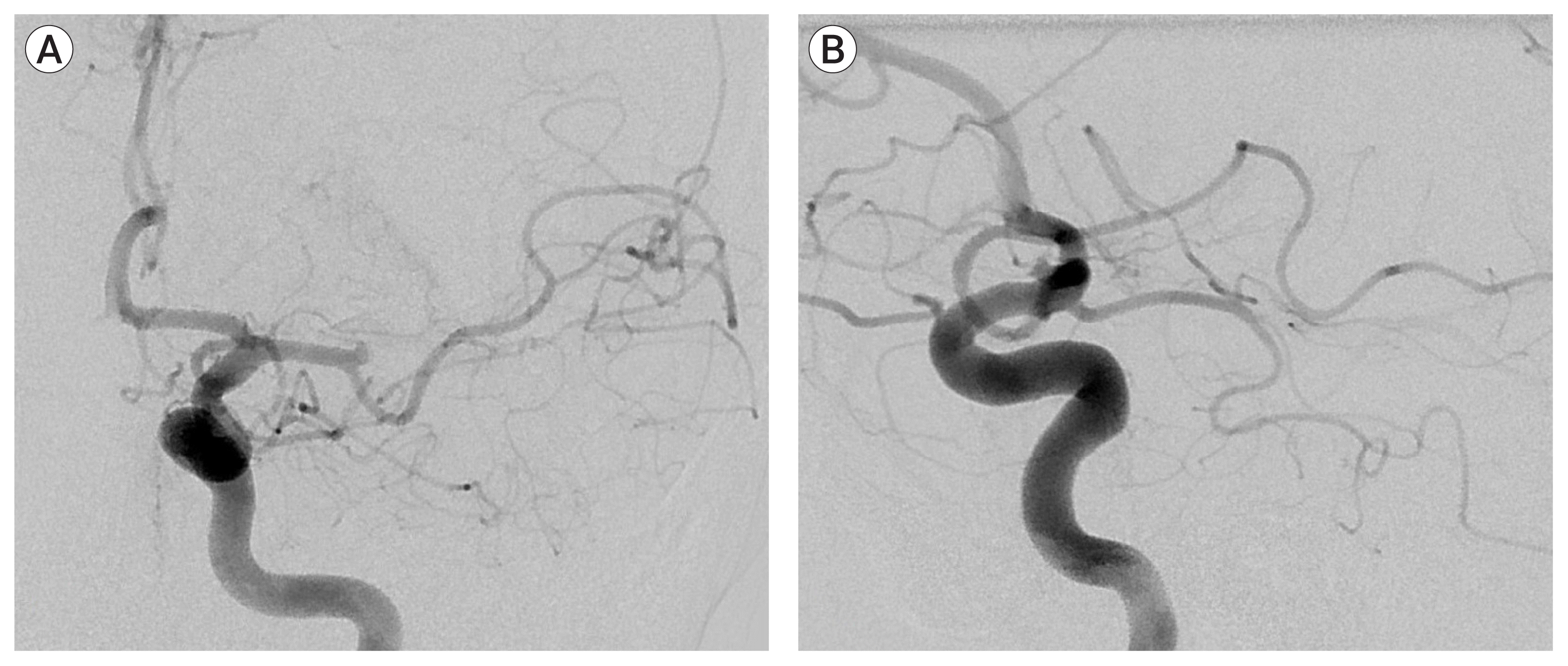
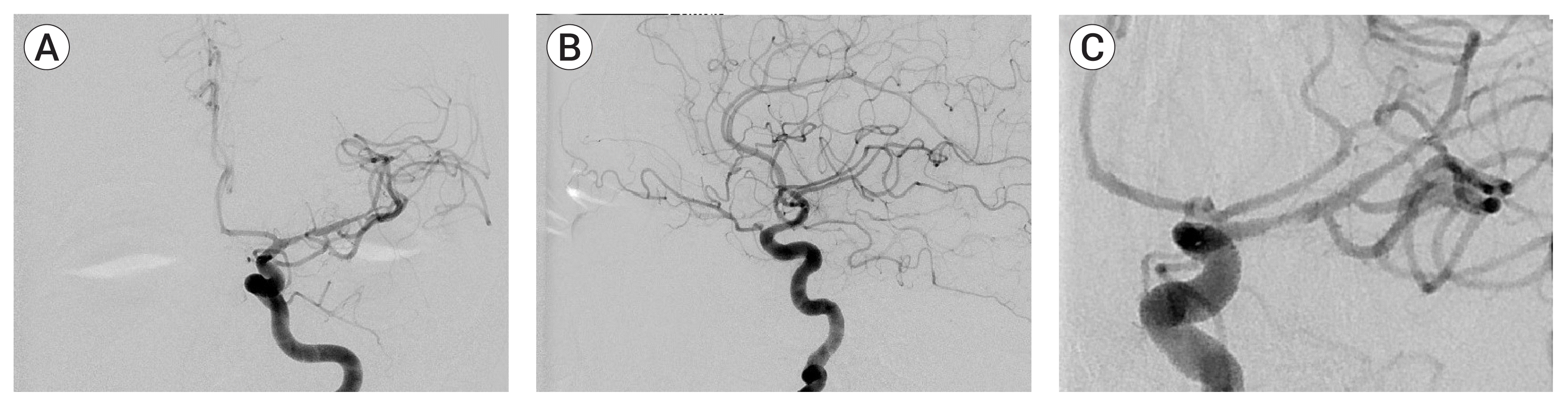
By the time he was taken the angiography suite, his NIHSS score had increased to 6. A cerebral angiogram under general endotracheal anesthesia confirmed total occlusion of superior trunk of the left middle cerebral artery (MCA) (Fig. 2). After ten attempts of thrombectomy with thromboaspiration and/or stentrievers, the thrombus was seen to migrate more proximally, into the M1 segment. As the clot was very hard to traverse, we opted to deploy a closed-cell intracranial stent (Enterprise, Codman & Shurtleff, Raynham, MA, USA) through a 0.021-inch microcatheter. The stent was partially deployed over the hard clot. After verification of the patency of M1 segment of MCA by an angiogram, it was fully deployed. The patient was started on a bolus of GP IIb/IIIa antagonist (tirofiban, 33 mL first 3 minutes, 12 mL/hr as maintenance infusion). Angiography showed a Thrombolysis in Cerebral Infarction (TICI) grade 3 reperfusion of the left MCA territory (Fig. 3).
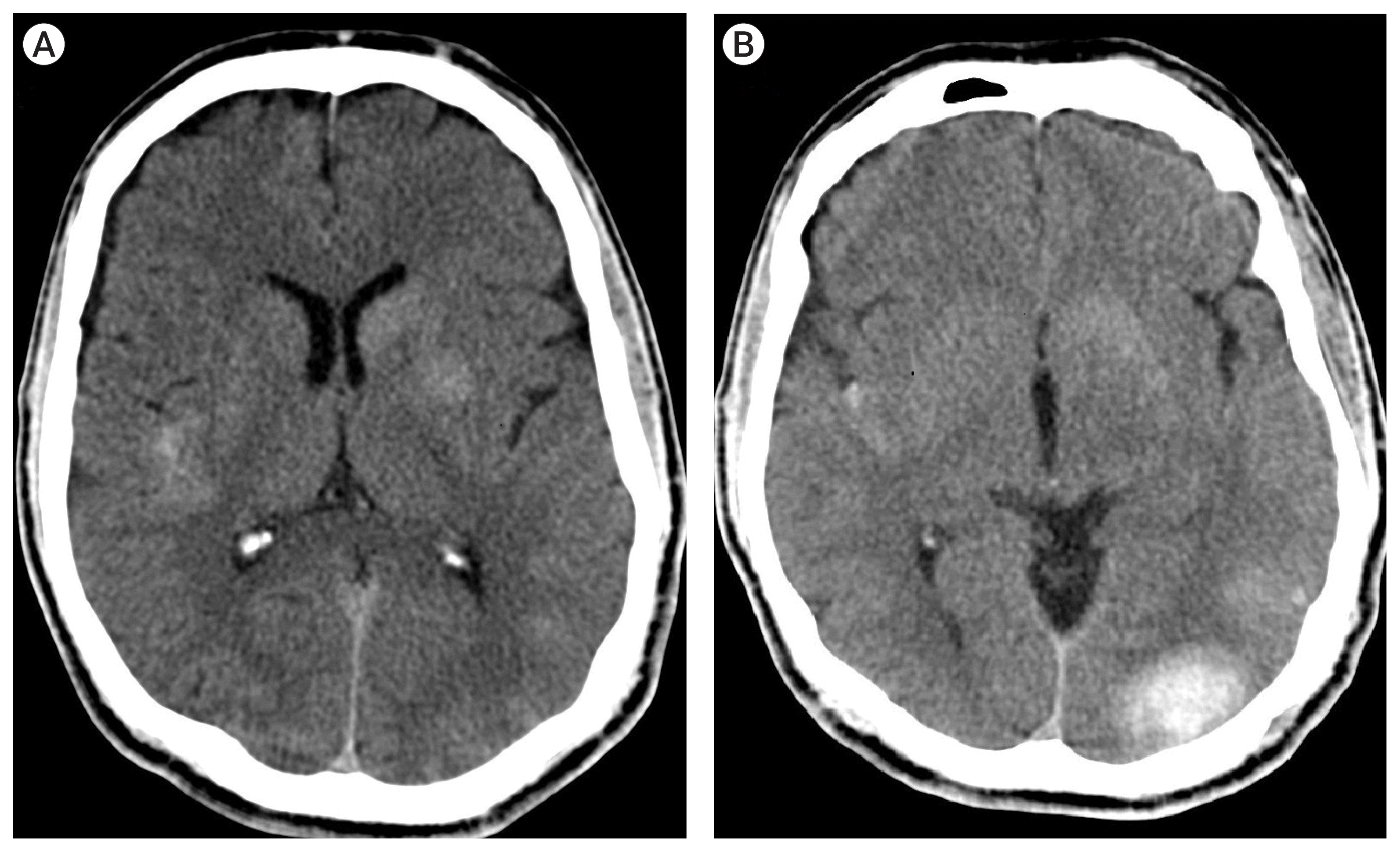
The post-procedural NIHSS score was 3. However a control brain CT without contrast obtained several hours after the procedure showed bilateral small intraparenchymal hemorrhagic lesions and a focal subarachnoid hemorrhage (Fig. 4). The laboratory workup revealed a white blood cell count of 13×10^3/μL, the erythrocyte sedimentation rate of 33 mm/hr and C-reactive protein level of 5.49 mg/dL. Transthoracic and transesophageal echocardiogram revealed a 17×5 mm vegetation on the ventricular aspect of the mitral valve and a diagnosis of infective endocarditis was made. Streptococcus sanguinis was identified on multiple sets of blood cultures and treatment with sulbactam/ampicillin and gentamicin was initiated. The patient was kept on IV tirofiban during follow-up and underwent valve replacement under tirofiban infusion on the fifth day of the endovascular procedure.
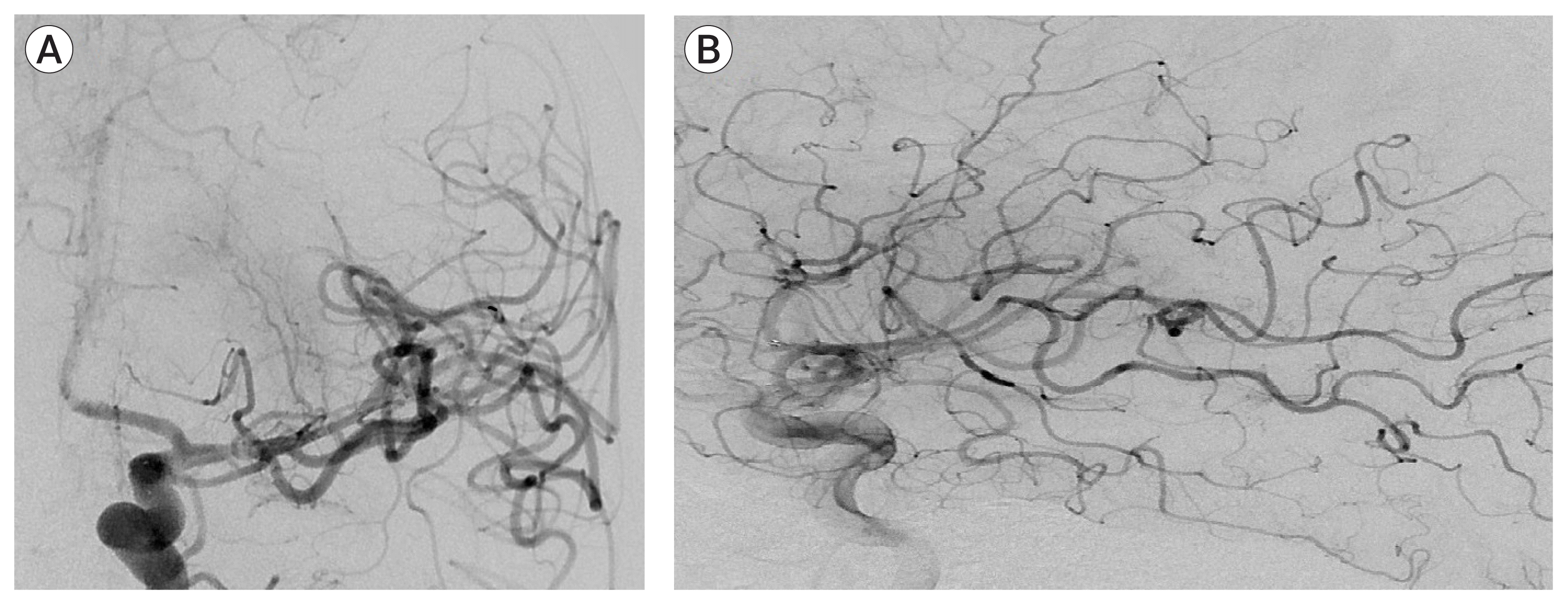
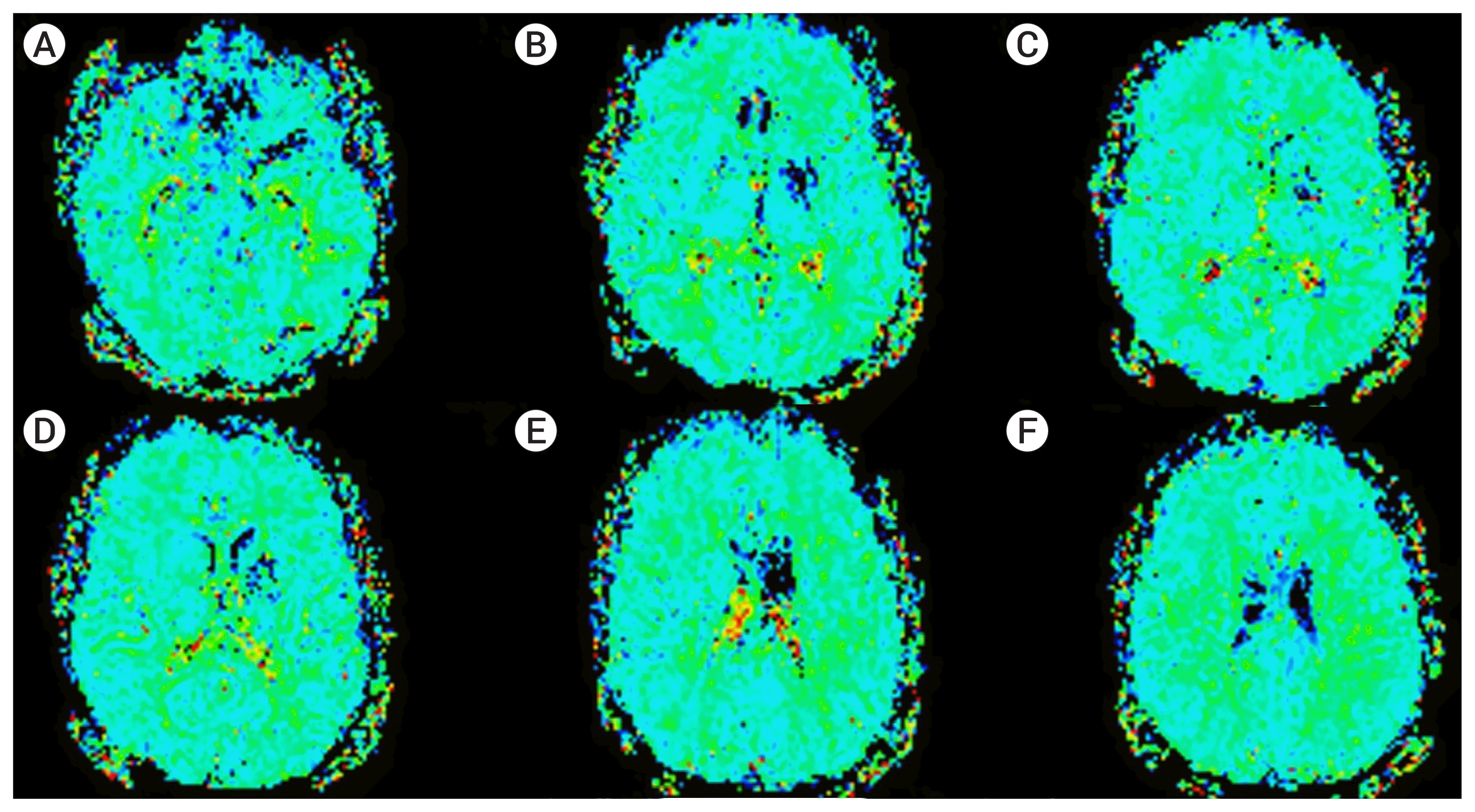
The patient was discharged with a NIHSS score of 0 after two weeks on clopidogrel and warfarin therapy. Two control angiograms at 2 months and 6 months showed stent patency with persistent residual stenosis (Fig. 5). The modified Rankin Scale (mRS) and NIHSS scores were 0 at 4 months respectively. Magnetic resonance (MR) perfusion imaging at 10 months showed a chronic infarct of the putamen and no significant perfusion asymmetry between two hemispheres (Fig. 6). Based on the results of the perfusion study and due to sthe patients asymptomatic status, we decided to follow the patient medically.
Go to : 
A review of literature revealed 26 cases of mechanical thrombectomy in IE related stroke. Twentythree were successful and in 3 the authors had to revert to intracranial stenting due to the failure of mechanical thrombectomy.1)2)5)8–12) Bain et al. used balloon expandable coronary stent, postulating that a self-expanding stent would not be sturdy enough to hold the firm clot against the arterial wall and maintain MCA patency.2) Hernandez-Fernandez et al. used a Solitaire (Medtronic, Dublin, Ireland) device for revascularization and stenting but they also had to perform an in-stent angioplasty to maintain patency during the same procedure.5) That is to say, a definitive stenting together with angioplasty was needed in more than 10% of the thrombectomy cases with acute IE.
Our review of the relevant literature also suggests that thrombectomy for embolic IE differs from routine thrombectomy in several aspects: To begin with, these patients are frequently anticoagulated and may have acute/subacute infarctions in unrelated cerebral territories. Consequently, there is an increased risk of intracranial hemorrhage both during the thrombectomy procedure and after the thrombectomy procedure. The likelihood of observing distal mycotic aneurysms is not low, further increasing the perioperative risk of hemorrhage. Last but not the least, previous reports have argued that the embolus in IE may be tighter due to a higher inflammatory content and compacted fibrotic nature, decreasing the likelihood of successful recanalization, especially when aspiration is not successful.2)5)
Our case is the first one in which only stenting was utilized for intracranial revascularization in IE. Consequently, residual stenosis occurred after intracranial stenting of the recalcitrant embolus.
However, during almost a year of follow-up, the patient was asymptomatic and imaging was unremarkable except for residual stenosis. MR perfusion imaging was interpreted to be normal. Contrary to the allegation of Bain et al.2), it was possible to achieve sufficient recanalization with a closed-cell intracranial stent to keep the patient asymptomatic. Arterial patency was sustained on the six-month digital subtraction angiography follow-up. Since it is not always possible to navigate a coronary stent intracranially and since acute post-stent angioplasty may be associated with a risk of arterial dissection or perforation, this method may be reserved as a rescue procedure for such intractable emboli. As we demonstrated, it is possible to test if the intracranial stent is strong enough to keep the artery patent by obtaining angiograms while the stent is half-deployed during the thrombectomy procedure. If the partial deployment fails to keep the artery patent, the stent can be resheated and a balloon-expandable coronary stent may then be considered.
Intracranial stenting is not risk-free. First of all, it alters the anticoagulation protocols in these patients. We used tirofiban during the procedure and for the next 5 days. As previously mentioned, both IE itself and a possible need for thoracic surgery impose the need for lifelong anticoagulation in these patients.9) In a single previously reported case, valve replacement was necessary due to the development of severe mitral insufficiency but unlike our case, the timing of surgery was not mentioned by the authors.5) In our case, tirofiban use complicated the management of the patient. Tirofiban is known to increase the risk of intracranial hemorrhage, especially in acute stroke cases like our patient.7) As a matter of fact, tirofiban is contraindicated in patients with intracranial hemorrhage due to a risk of exacerbation of the bleeding.4) Although our patient had a post procedure intracranial bleeding, we were unable to discontinue tirofiban (we took the risk of hematoma expansion) since he already had an intracranial stent in place. Secondly, as a previously overlooked concern, there is a theoretical possibility that placement of a foreign body (stent) over a possibly infected embolus in a an acutely septic patient might be risky in terms of arterial patency and integrity. As the case by Bain et al.2) did not undergo follow-up vascular imaging, our case and a case reported by Hernandez-Fernandez et al. are the only cases that demonstrate the absence of arterial wall involvement by an infectious process on follow-up.5) Perioperative antibiotic coverage is critical for febrile patients presenting for intracranial thrombectomy to avoid a potentially fatal arterial damage on follow-up.
Go to : 
A recent history of febrile status or presentation with fever, as we noted in our patient, should raise a suspicion of IE as a cause of acute stroke. Due to time constraints, a workup (e.g. transesophageal ultrasound) may not be possible in this setting. The operator may consider cardiologic evaluation on the angiography suite, or as an alternative may choose to start the procedure with thromboaspiration, keeping in mind the condensed nature of the clot and reserve intracranial stent placement for resistant cases for reasons we described.
Go to : 
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Go to : 
REFERENCES
1. Ambrosioni J, Urra X, Hernandez-Meneses M, Almela M, Falces C, Tellez A, et al. Mechanical Thrombectomy for Acute Ischemic Stroke Secondary to Infective Endocarditis. Clin Infect Dis. 2018; Apr. 66(8):1286–9.

2. Bain MD, Hussain MS, Gonugunta V, Katzan I, Gupta R. Successful recanalization of a septic embolus with a balloon mounted stent after failed mechanical thrombectomy. J Neuroimaging. 2011; Apr. 21(2):170–2.

3. Bhuva P, Kuo SH, Claude Hemphill J, Lopez GA. Intracranial hemorrhage following thrombolytic use for stroke caused by infective endocarditis. Neurocrit Care. 2010; Feb. 12(1):79–82.

4. Bukow SC, Daffertshofer M, Hennerici MG. Tirofiban for the treatment of ischaemic stroke. Expert Opin Pharmacother. 2006; Jan. 7(1):73–9.

5. Hernandez-Fernandez F, Rojas-Bartolome L, Garcia-Garcia J, Ayo-Martin O, Molina-Nuevo JD, Barbella-Aponte RA, et al. Histopathological and bacteriological analysis of thrombus material extracted during mechanical thrombectomy in acute stroke patients. Cardiovasc Intervent Radiol. 2017; Dec. 40(12):1851–60.

6. Junna M, Lin CC, Espinosa RE, Rabinstein AA. Successful intravenous thrombolysis in ischemic stroke caused by infective endocarditis. Neurocrit Care. 2007; 6(2):117–20.

7. Kellert L, Hametner C, Rohde S, Bendszus M, Hacke W, Ringleb P, et al. Endovascular stroke therapy: tirofiban is associated with risk of fatal intracerebral hemorrhage and poor outcome. Stroke. 2013; May. 44(5):1453–5.

8. Kim JM, Jeon JS, Kim YW, Kang DH, Hwang YH, Kim YS. Forced arterial suction thrombectomy of septic embolic middle cerebral artery occlusion due to infective endocarditis: an illustrative case and review of the literature. Neurointervention. 2014; Sep. 9(2):101–5.

9. Ladner TR, Davis BJ, He L, Kirshner HS, Froehler MT, Mocco J. Complex decision-making in stroke: preoperative mechanical thrombectomy of septic embolus for emergency cardiac valve surgery. J Neurointerv Surg. 2015; Dec. 7(12):e41.

10. Scharf EL, Chakraborty T, Rabinstein A, Miranpuri AS. Endovascular management of cerebral septic embolism: three recent cases and review of the literature. J Neurointerv Surg. 2017; May. 9(5):463–5.

11. Sloane KL, Raymond SB, Rabinov JD, Singhal AB. Mechanical thrombectomy in stroke from infective endocarditis: case report and review. J Stroke Cerebrovasc Dis. 2019; Jan. 29(1):104501.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download