Abstract
Objective
The role of surgery in spontaneous intracerebral hemorrhage (sICH) is still controversial. We aimed to investigate the effectiveness of minimally invasive surgery (MIS) compared to conventional surgery (CS) for supratentorial sICH.
Methods
The medical data of 70 patients with surgically treated supratentorial sICH were retrospectively reviewed. MIS was performed in 35 patients, and CS was performed in 35 patients. The surgical technique was selected based on the neurological status and radiological findings, such as hematoma volume, neurological status and spot signs on computed tomographic angiography. Treatment outcomes, prognostic factors and the usefulness of the spot sign were analyzed.
Results
Clinical states in both groups were statistically similar, preoperatively, and in 1 and 3 months after surgery. Both groups showed significant progressive improvement till 3 months after surgery. Better preoperative neurological status, more hematoma removal and intensive care unit (ICU) stay ≤7 days were the significant prognostic factors for favorable 3-month clinical outcomes (odds ratio [OR] 0.31, 95% confidence interval [CI] 0.10–0.96, p=0.04; OR 1.04, 95% CI 1.01–1.08, p=0.02; OR 26.31, 95% CI 2.46–280.95, p=0.01, respectively). Initial hematoma volume and MIS were significant prognostic factors for a short ICU stay (≤7 days; OR 0.95; 95% CI 0.91–0.99; p=0.01; OR 3.91, 95% CI 1.03–14.82, p=0.045, respectively). No patients in the MIS group experienced hematoma expansion before surgery or postoperative rebleeding.
Go to : 
Spontaneous intracerebral hemorrhage (sICH) accounts for approximately 15–24% of all strokes.3) The sICH is the second most common subtype of strokes and leads to severe disability or death.7) Theoretically, surgical hematoma removal may reduce mass effects and secondary brain injuries by relieving perilesional edema and removing noxious chemicals.7)9)18) However, the effectiveness of surgery for sICH remains controversial.8)9)18) A few randomized trials failed to prove a role for surgery in improving clinical outcomes in patients with low to moderate amounts of hemorrhage, and it has been pointed out that conventional craniotomy can cause additional brain injury.8)9)18) Recently, minimally invasive surgery (MIS), including endoscopic surgery and stereotactic or navigator-guided aspiration with chemical or sonographic thrombolysis, has been attempted in order to minimize additional brain injuries caused by surgery itself and has shown satisfactory outcomes.12)13)15)16)23)24) Compared to conventional surgery (CS) and medical management, MIS has some advantages, such as a shorter operation time and stay in the intensive care unit (ICU), better cost-effectiveness, less fatigue associated with medical teams and less invasiveness for patients.2) Meanwhile, with MIS, it is usually difficult to remove the entire hematoma and control the intraoperative bleeding from perforators unless there is an endoscopic system.2)22) Therefore, selection of the appropriate surgical technique between MIS and CS is another important issue. The purpose of this study is to investigate the effectiveness of MIS compared with CS and the usefulness of spot sign on computed tomographic (CT) angiography for predicting hematoma expansion and rebleeding as well as to determine the surgical technique in patients with supratentorial sICH.
Go to : 
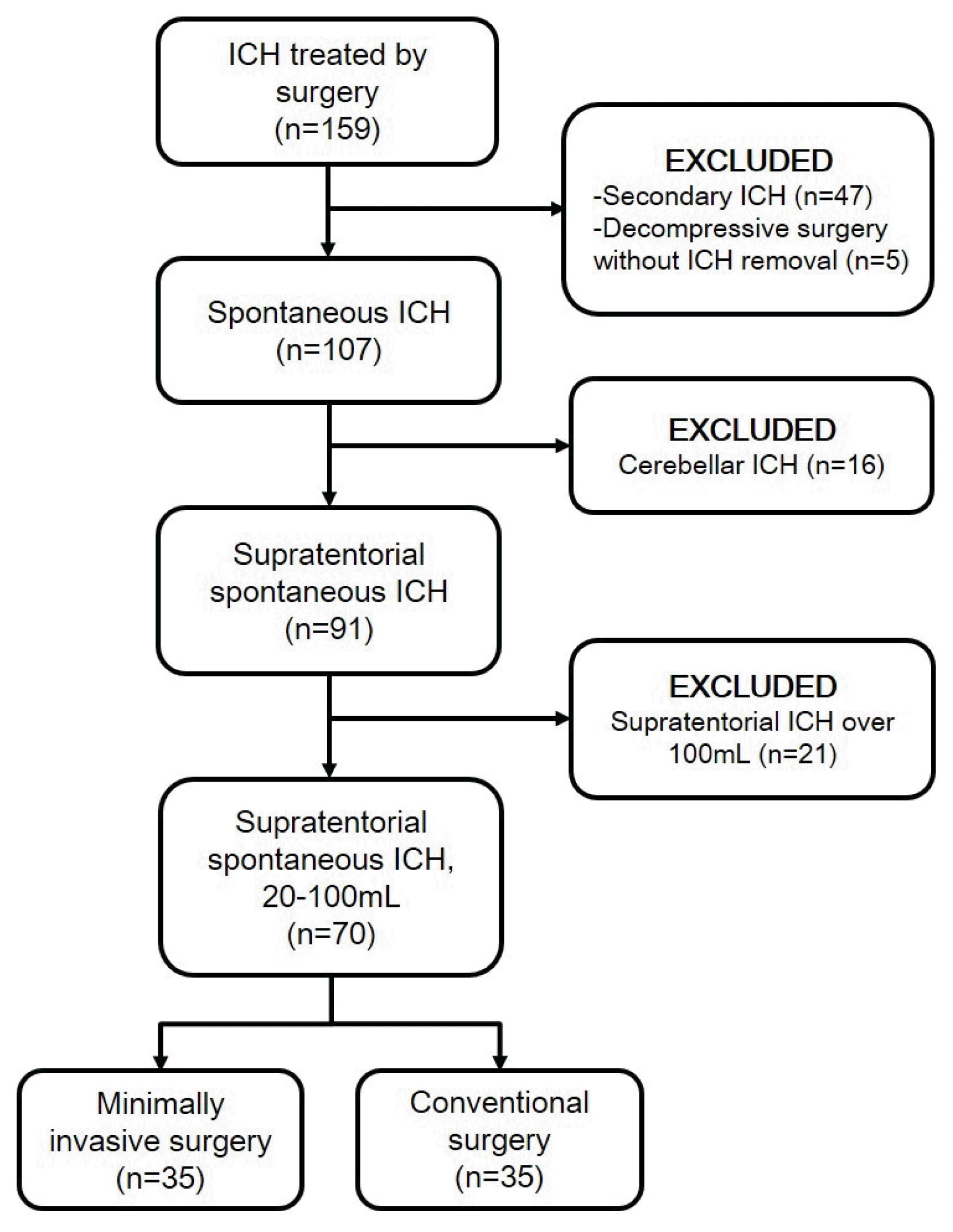
We retrospectively reviewed 159 patients who underwent surgery for sICH between April 2010 and April 2019 under the approval of the institutional review board. Among them, there were 47 patients with secondary ICH caused by cerebral aneurysms, vascular malformations, moyamoya diseases and tumors, and 5 who underwent decompressive surgery without hematoma evacuation and 16 with cerebellar ICH were excluded. Of the 91 patients, 21 patients with supratentorial ICH more than 100 mL were excluded. Finally, 70 patients with supratentorial sICH were enrolled in this study, and they were divided into 2 groups: the MIS (n=35) and CS (n=35) groups (Fig. 1).
Surgical intervention, including CS and MIS, was performed in patients with the following conditions: 1) supratentorial sICH; 2) hematoma volume between 20 and 100 mL; 3) age of 18 years or more; and 4) neurological deterioration and Glasgow coma scale of 14 or less. The operative technique was generally determined according to the following protocol. When ICH was detected on initial CT or magnetic resonance imaging, CT angiography was subsequently performed to determine spot signs and some causes of bleeding, such as cerebrovascular diseases and brain tumors. Patients with massive bleeding (>100 mL), brain swelling and neurological deterioration underwent emergent CS. Patients with a hematoma volume less than 100 mL, those with no or mild midline shifting and brain swelling on initial CT, or those who had an alert, drowsy or stupor mental status underwent follow-up CT within 4 hours from initial presentation to evaluate hematoma progression. CS was selected in patients with positive spot signs, hematoma progression, brain swelling and progressive neurological deterioration. MIS was performed in others with no evidence of hematoma expansion, no or minimal progression of brain swelling on follow-up CT, and no or minimal change in mental status.
MIS was conducted via burr hole trephination, catheter insertion toward the center of the hematoma and manual aspiration of the hematoma with several 10 mL syringes, with the aid of stereotactic or navigation systems under local or general anesthesia. MIS aimed to decrease hematoma burden, consequently relieving perilesional edema and controlling intracranial cerebral pressure (ICP). The drainage catheter within the hematoma cavity was removed 2 or 3 days after the surgery. CS included conventional craniotomy and decompressive craniectomy under general anesthesia. The aim of CS was not only to remove the hematoma and relieve ICP but also to find and coagulate the bleeding culprit. According to the degree of brain swelling, craniectomy, duroplasty and lobectomy were additionally performed.
The patients’ baseline characteristics are summarized in Table 1. Clinical status was evaluated with the Glasgow Coma scale and modified Rankin scale (mRS).17) There were no significant differences between the 2 groups in terms of age, sex, underlying diseases, smoking, antithrombotic use, and preoperative clinical status. However, patients in the MIS group had a smaller initial hematoma volume than patients in the CS group.
Baseline characteristics and surgical outcomes
CT scans were performed preoperatively, immediately after surgery and when necessary. The hematoma volume was estimated by summing the volumes of each CT slice (area of hemorrhage in each slice [cm2]×thickness of each slice [0.3 or 0.5 cm]). The presence of spot signs and tiny enhancing foci within the hematoma on CT angiography was evaluated by a radiologist and neurosurgeon.20)
Statistical analyses were performed with IBM SPSS Statistics for Windows Version 25.0 (IBM Corp., Armonk, NY, USA). Continuous variables are expressed as the mean±standard deviation (range), and categorical variables are expressed as the number of patients (percentage). Mann-Whitney U, Pearson’s Chi-squared and Fisher’s exact tests were used for the comparison of baseline characteristics and outcomes between the 2 groups. For the comparison of serial mRS scores, the Wilcoxon signed rank test was adopted. The prognostic factors of a favorable clinical outcome (postoperative 3-month mRS ≤3) and short ICU stay (≤7 days) were assessed with univariate and multivariate logistic regression models. Prognostic factors with a p value <0.20 in the univariate analysis were selected for the multivariate analysis. A p value <0.05 was considered statistically significant.
Go to : 
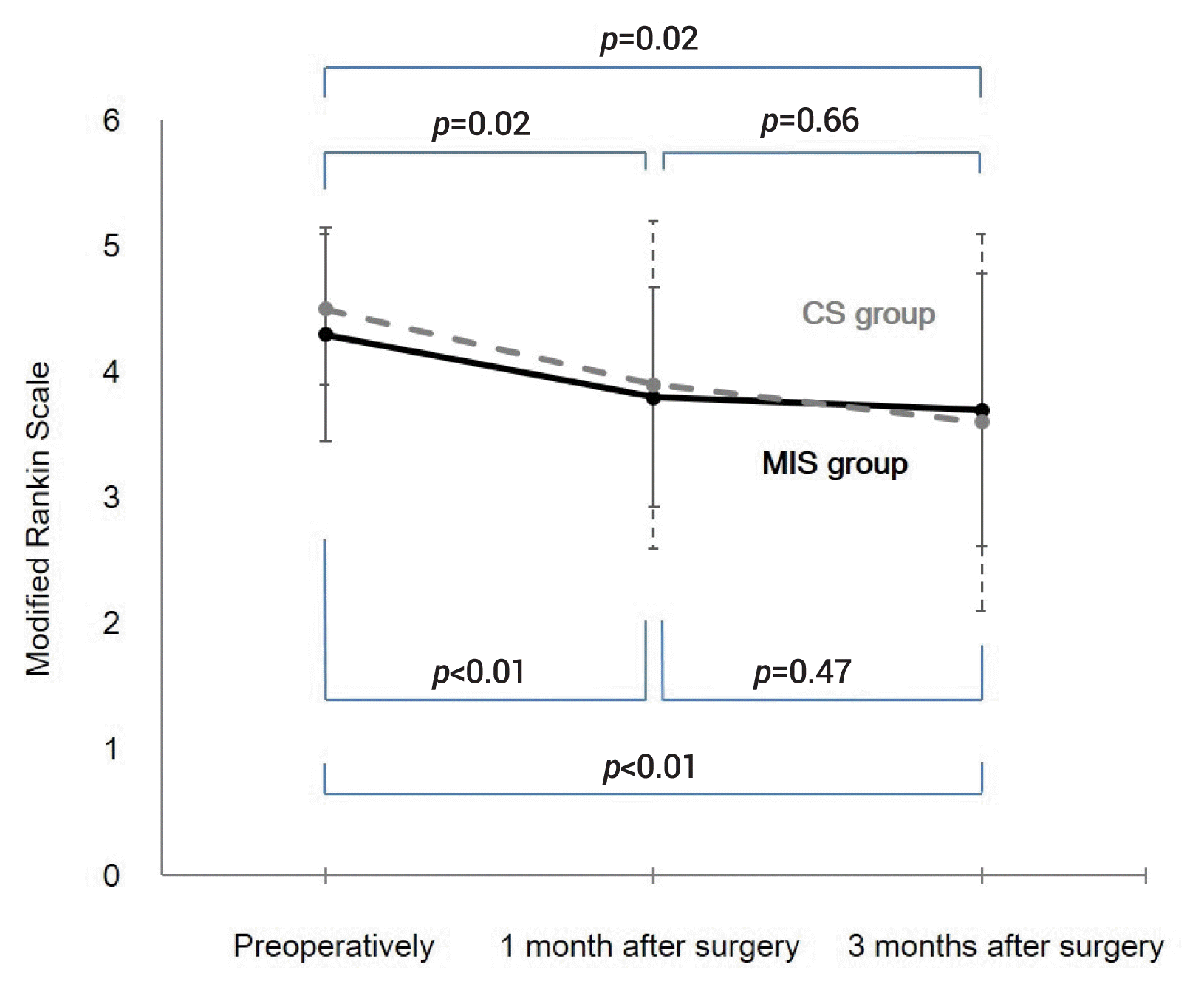
The clinical states in the preoperative and postoperative periods are presented in Table 1 and Fig. 2. Irrespective of group, the clinical status progressively improved in terms of mRS scores from the preoperative period to the 3-month postoperative period, with a significant difference (Fig. 2).
The comparison of the mRS scores at each time period between the 2 groups was not significant. The 1-month and 3-month mortality rates were similar in both groups (2.9% vs. 11.4%; p=0.36, 2.9% vs. 14.3%; p=0.20, respectively).
In the multivariate analysis (Table 2), better preoperative neurological status, higher amount of removed hematoma volume and a short stay in the ICU of 7 days or less were significant prognostic factors for the favorable 3-month clinical outcomes (mRS ≤3; odds ratio [OR] 0.31, 95% confidence interval [CI] 0.10–0.96, p=0.04; OR 1.04, 95% CI 1.01–1.08, p=0.02; OR 26.31, 95% CI 2.46–280.95, p=0.01, respectively).
Univariate and multivariate analyses for prognostic factors for favorable clinical outcomes*
The MIS group had a significantly shorter ICU stay (6.4±6.5 days vs. 13.0±13.2 days; p<0.01) than the CS group, however, ICU-related complications occurred similarly in both groups (Table 1). The multivariate analyses demonstrated that initial hematoma volume and MIS as operation technique (over CS) were significant prognostic factors for a short ICU stay (≤7 days; OR 0.95; 95% CI 0.91–0.99; p=0.01; OR 3.91, 95% CI 1.03–14.82, p=0.045, respectively) (Table 3).
Univariate and multivariate analyses for prognostic factors for short stay at intensive care units*
Patients in the MIS group had a smaller preoperative hematoma volume than patients in the CS group (54.8±19.3 mL vs. 68.4±18.1 mL; p<0.01), and the hematoma evacuation rate was lower in the MIS group than in the CS group (46.2±26.6% vs. 76.5±41.8%; p<0.01) (Table 1). Two patients (5.7%) in the CS group showed symptomatic rebleeding after surgery, and all of them underwent repeat operations.
Among the 70 patients, 42 patients (60%) underwent initial CT angiography, and the spot sign was observed in 7 patients (Table 4). Of the 7 patients with positive spot signs, 2 patients underwent MIS because there was no hematoma expansion detected on follow-up CT. The other 5 patients underwent CS, among whom 3 showed hematoma expansion on follow-up CT. One of 3 patients in the CS group showed postoperative rebleeding, whereas there were no cases of rebleeding in the MIS group. Of the 35 patients with a negative spot sign, 25 with no evidence of hematoma expansion on follow-up CT underwent MIS, and the other 10 with poor neurological status received CS. There were no cases of rebleeding after surgery in the MIS group; however, 1 case of rebleeding occurred after surgery in the CS group.
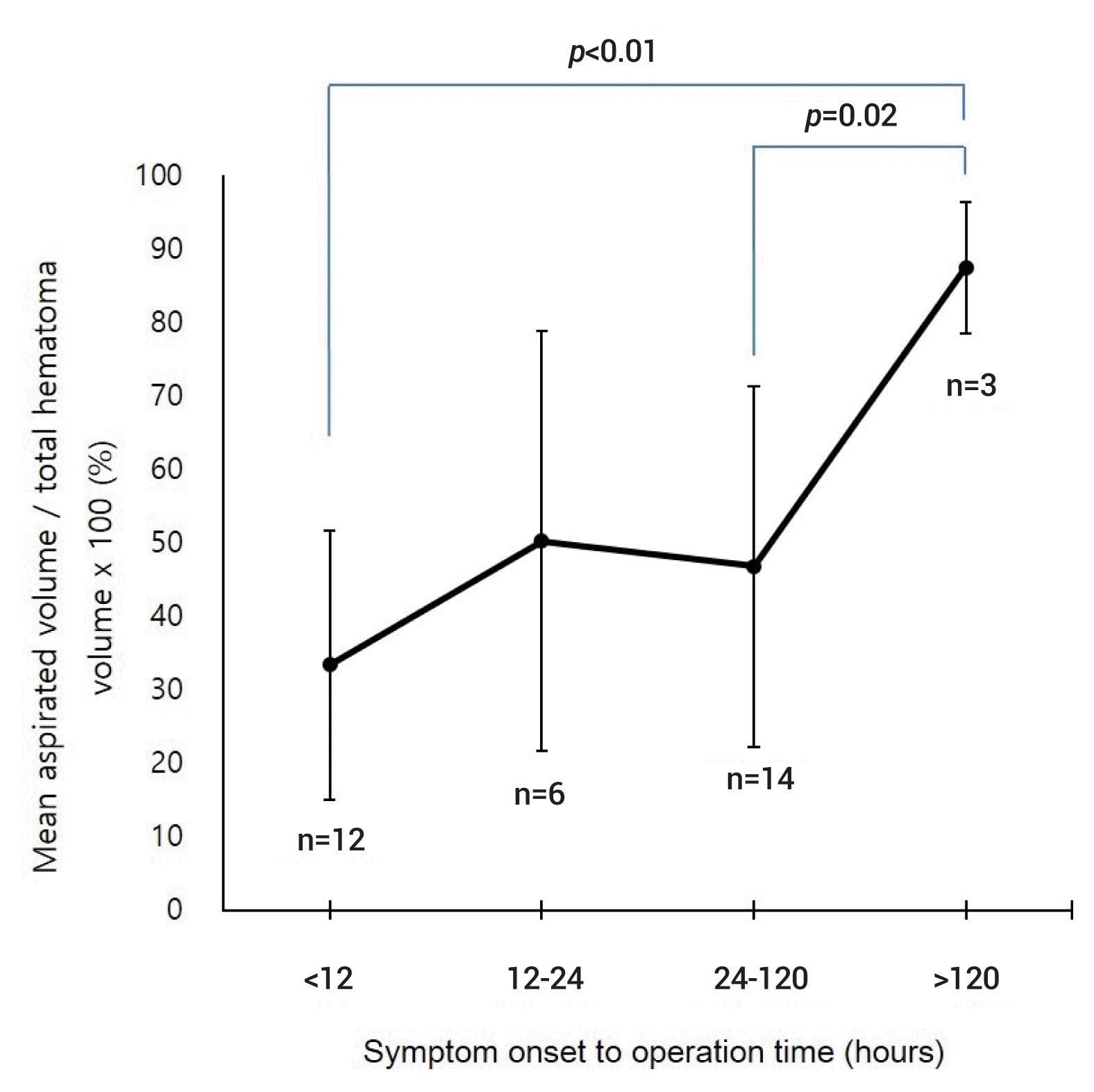
The aspirated volume during MIS was compared according to the surgical timing from symptom onset (Fig. 3). Among 35 patients who underwent MIS, 12 underwent surgery within 12 hours, 6 between 12 and 24 hours, 14 between 24 and 120 hours, and 3 after 120 hours from symptom onset. MIS after 120 hours (87.4±8.9% [80.0–100.0%]) demonstrated the highest efficiency in terms of hematoma aspiration compared with surgery at the other time periods (33.3±18.4% [2.7–58.5%] within 12 hours, 50.2±28.6% [11.5–94.2%] between 12 and 24 hours and 46.8±24.5% [3.3–100%] between 24 and 120 hours).
Go to : 
There have been several large randomized controlled trials comparing surgery and conservative treatment for supratentorial sICH, and the outcomes were neutral because of the data heterogeneity.8)9) The supratentorial intracerebral haematomas in the international surgical trial in intracerebral haemorrhage (STICH) I trial, the first large trial regarding supratentorial ICH with a volume of 10–100 mL, showed that early craniotomy had no overall benefit in terms of mortality and long-term functional outcomes compared with conservative treatment. However, a subgroup analysis demonstrated a potential benefit of early surgery for lobar hemorrhage located at a depth <1 cm from the surface.8) However, the STICH II trial, which launched based on the subgroup analysis from the STICH I trial, also failed to demonstrate a difference.9) In order to minimize conventional craniotomy and craniectomy-related brain injuries, minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE) trials were designed to compare MIS and medical treatment in groups with a moderate volume of supratentorial sICH. The MISTIE II trial provided some evidences of safety, no differences in 30-day mortality or symptomatic rebleeding, and a promising treatment effect whereby mRS scores=0–3 (>10% absolute benefit) were sustained for 1 year.4) In the subsequent MISTIE III trial, the surgical treatment did not improve functional outcomes; however, the 6-month and 1-year mortality rates were lower in the MIS group.5) In addition, hematoma evacuation was associated with a significant reduction in perilesional edema.11) Based on these previous trials, MIS seems to be more beneficial than conventional craniotomy and medical treatment for patients with sICH.
There have been many retrospective and prospective comparative studies regarding MIS and CS.2)12)15)16)22–24) A meta-analysis using only randomized controlled trials reported that MIS was superior to CS or medical management in terms of death or dependence at the end of follow-up.24) Another meta-analytic study with randomized controlled trials and some prospective trials demonstrated that MIS was better than CS regarding mortality, good functional outcomes and postoperative rebleeding events.22) Another meta-analysis with a combination of prospective and retrospective studies showed better functional outcomes and hematoma evacuation rates and lower rates of rebleeding and mortality as well as operation times, amounts of intraoperative blood loss, ICU stays, hospital days, and postoperative infections and complications.15) In this retrospective study, the clinical outcomes were similar in the 2 groups; however, the ICU stay was shorter in the MIS group than in the CS group, and there were no cases of rebleeding in the MIS group. Moreover, MIS was one of the significant prognostic factors for a short ICU stay which was also one of the significant factors for the favorable clinical outcomes. MIS, at least, seems to not be inferior to CS.
In our institution, operative techniques are determined based on hematoma volume, brain swelling, midline shifting, hematoma softness, ability to control the bleeding focus and neurological status. Some groups recommend the use of MIS with hematoma volumes less than 80 mL;2) however, the important factors for determining surgical techniques include not only the hematoma volume but also ICP control. In fact, although not included in this study, the largest hematoma volume in the MIS group was 121 mL, in which the patient’s ICP was well controlled by removing the hematoma without additional medical support to reduce the ICP. Conversely, in patients with a moderate amount of hematoma, but high ICP and poor neurological status, CS is more appropriate than MIS. One of the important selection criteria for MIS is the possibility of rebleeding. Rebleeding is known to be related to a poor functional outcome.14) The authors of this study performed simple hematoma aspiration based on the spot sign detected on CT angiography as well as hematoma expansion detected on follow-up CT. The spot sign is well known to be positively associated with hematoma expansion and is useful to predict intraoperative and postoperative rebleeding.1)10)19) None of the patients with a negative spot sign in the MIS group had rebleeding, and rebleeding occurred only in the CS group. The spot sign seems to be a useful radiological marker for determining the operation technique as well as for predicting rebleeding. For patients with a high risk of rebleeding and hematoma expansion, CS or MIS with a neuroendoscopic system capable of controlling bleeding should be considered.
The hematoma evacuation rate and its clinical meaning are still inconclusive. In this study, approximately 77% and 46% of the hematoma was removed in the CS and MIS groups, respectively. A low rate of hematoma evacuation is a drawback of simple aspiration techniques used in MIS. Actually, removed hematoma volume was analyzed one of significant prognostic factors for favorable clinical outcome although there was no difference in the clinical outcomes between the 2 groups. A randomized controlled study and meta-analysis reported that the hematoma evacuation rate was lower with simple aspiration than with neuroendoscopic MIS and CS; however, there was no analysis of the hematoma evacuation rate and its clinical implication.2)15) In the MISTIE III trial,5) simple aspiration with alteplase thrombolysis achieved approximately 70% evacuation of the initial hematoma. Clot size reduction to 15 mL or less was related to better 1-year functional outcomes. In addition, the volume of the remaining hematoma was positively associated with the extent of perilesional edema,11) and a recent study showed that growth of perilesional edema was related to a high 6-month mortality rate.21) Although further studies are needed, effective MIS techniques such as the use of a neuroendoscope should be considered to increase the evacuation rate without additional risks and to improve clinical outcomes.
Optimal surgical timing can be important in terms of rebleeding risks and secondary brain injuries. Recently, there have been contradictory opinions about early surgery, which increases the risk of rebleeding versus improves the prognosis by reducing secondary insults.20)23) A subgroup analysis in the STICH II trial demonstrated that patients operated on before 21 hours from ictus had a trend towards a better outcome.9) Clinical guidelines from the American Stroke Association suggest that early surgery (within 8 hours after hemorrhage) improves outcomes and that ultra-early surgery (within 4 hours from ictus) is associated with an increased risk of rebleeding.6) The optimal timing of MIS for sICH has not been established. A meta-analysis suggested that MIS showed a significantly better clinical outcome, lower mortality rate and higher hematoma evacuation rate than CS within 24 hours.16) With the concept that ultra-early surgery can cause rebleeding, and that delayed surgery cannot achieve a high hematoma evacuation rate due to clot hardening and a favorable clinical outcome due to progression of secondary injury, the authors tried to perform MIS between 6 and 12 hours from symptom onset. In this study, however, there were no such differences within 120 hours, and the hematoma evacuation rate beyond 120 hours from symptom onset was the highest. In addition, there was no rebleeding case in the MIS group. Prospective studies are needed to confirm the optimal operation timing, and MIS with advanced tools such as neuroendoscopes and ultrasound is expected to safely remove sufficient hematoma volumes, irrespective of rebleeding risk and clot hardness.13)15)
Our study had some limitations. First, it was a retrospective, small-sized and single-center study. Second, preoperative CT angiography data were incomplete. However, approximately 80% of patients in the MIS group had preoperative CT angiography data, and this was sufficient to confirm the usefulness of the spot sign for selecting the surgical technique and predicting rebleeding.
Go to : 
Although baseline characteristics such as the initial hematoma volume were different between the 2 groups, MIS was not inferior to CS in terms of clinical outcomes. In addition, the ICU stay was shorter, and there were no cases of rebleeding after surgery in the MIS group, compared with the CS group. In the multivariate analysis, more hematoma removal, better initial neurological status and short ICU stay were prognostic factors for favorable 3-month clinical outcomes, and initial hematoma volume and MIS were the significant factors for a short ICU stay. Therefore, MIS is considered an effective surgical technique for sICH. As the patients with a negative spot sign depicted on initial CT angiography showed no evidence of hematoma expansion during the follow-up before surgery and as there were no cases of rebleeding after surgery in the MIS group, the spot sign can be useful for determining the surgical method as well as for predicting hematoma expansion, which is already well known.20) Further randomized and prospective studies will provide more significant and confirmed information.
Go to : 
Notes
Disclosure
The authors report no conflict of interest concerning the materials or methods used in this study or the findings specified in this paper.
Go to : 
REFERENCES
1. Brouwers HB, Raffeld MR, van Nieuwenhuizen KM, Falcone GJ, Ayres AM, McNamara KA, et al. CT angiography spot sign in intracerebral hemorrhage predicts active bleeding during surgery. Neurology. 2014; Sep. 83(10):883–9.

2. Cho DY, Chen CC, Chang CS, Lee WY, Tso M. Endoscopic surgery for spontaneous basal ganglia hemorrhage: comparing endoscopic surgery, stereotactic aspiration, and craniotomy in noncomatose patients. Surg Neurol. 2006; Jun. 65(6):547–55.

3. Feigin VL, Lawes CM, Bennett DA, Barker-Collo SL, Parag V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol. 2009; Apr. 8(4):355–69.

4. Hanley DF, Thompson RE, Muschelli J, Rosenblum M, McBee N, Lane K, et al. Safety and efficacy of minimally invasive surgery plus alteplase in intracerebral haemorrhage evacuation (MISTIE): a randomised, controlled, open-label, phase 2 trial. Lancet Neurol. 2016; Nov. 15(12):1228–37.

5. Hanley DF, Thompson RE, Rosenblum M, Yenokyan G, Lane K, McBee N, et al. Efficacy and safety of minimally invasive surgery with thrombolysis in intracerebral haemorrhage evacuation (MISTIE III): a randomised, controlled, open-label, blinded endpoint phase 3 trial. Lancet. 2019; Mar. 393(10175):1021–32.
6. Hemphill JC 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, et al. Guidelines for the Management of Spontaneous Intracerebral Hemorrhage: A Guideline for Healthcare Professionals from the American Heart Association/American Stroke Association. Stroke. 2015; Jul. 46(7):2032–60.
7. Keep RF, Hua Y, Xi G. Intracerebral haemorrhage: mechanisms of injury and therapeutic targets. Lancet Neurol. 2012; Aug. 11(8):720–31.

8. Mendelow AD, Gregson BA, Fernandes HM, Murray GD, Teasdale GM, Hope DT, et al. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial intracerebral haematomas in the International Surgical Trial in Intracerebral Haemorrhage (STICH): a randomised trial. Lancet. 2005; Jan. 365(9457):387–97.

9. Mendelow AD, Gregson BA, Rowan EN, Murray GD, Gholkar A, Mitchell PM. Early surgery versus initial conservative treatment in patients with spontaneous supratentorial lobar intracerebral haematomas (STICH II): a randomised trial. Lancet. 2013; Aug. 382(9890):397–408.

10. Miki K, Yagi K, Nonaka M, Iwaasa M, Abe H, Morishita T, et al. Spot sign as a predictor of rebleeding after endoscopic surgery for intracerebral hemorrhage. J Neurosurg. 2019; May. 130(5):1485–90.

11. Mould WA, Carhuapoma JR, Muschelli J, Lane K, Morgan TC, McBee NA, et al. Minimally invasive surgery plus recombinant tissue-type plasminogen activator for intracerebral hemorrhage evacuation decreases perihematomal edema. Stroke. 2013; Mar. 44(3):627–34.

12. Nam TM, Kim YZ. A meta-analysis for evaluating efficacy of neuroendoscopic surgery versus craniotomy for supratentorial hypertensive intracerebral hemorrhage. J Cerebrovasc Endovasc Neurosurg. 2019; Mar. 21(1):11–7.

13. Newell DW, Shah MM, Wilcox R, Hansmann DR, Melnychuk E, Muschelli J, et al. Minimally invasive evacuation of spontaneous intracerebral hemorrhage using sonothrombolysis. J Neurosurg. 2011; Sep. 115(3):592–601.

14. Ren Y, Zheng J, Liu X, Li H, You C. Risk factors of rehemorrhage in postoperative patients with spontaneous intracerebral hemorrhage: a case-control study. J Korean Neurosurg Soc. 2018; Jan. 61(1):35–41.
15. Sun S, Li Y, Zhang H, Gao H, Zhou X, Xu Y, et al. Neuroendoscopic surgery versus craniotomy for supratentorial hypertensive intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg. 2020; Feb. 134:477–88.

16. Tang Y, Yin F, Fu D, Gao X, Lv Z, Li X. Efficacy and safety of minimal invasive surgery treatment in hypertensive intracerebral hemorrhage: a systematic review and meta-analysis. BMC Neurol. 2018; Sep. 18(1):136.

17. Uyttenboogaart M, Stewart RE, Vroomen PC, De Keyser J, Luijckx GJ. Optimizing cutoff scores for the Barthel index and the modified Rankin scale for defining outcome in acute stroke trials. Stroke. 2005; Sep. 36(9):1984–7.

18. Vespa PM, Martin N, Zuccarello M, Awad I, Hanley DF. Surgical trials in intracerebral hemorrhage. Stroke. 2013; Jun. 44(6 Suppl 1):S79–82.

19. Wada R, Aviv RI, Fox AJ, Sahlas DJ, Gladstone DJ, Tomlinson G, et al. CT angiography “spot sign” predicts hematoma expansion in acute intracerebral hemorrhage. Stroke. 2007; Apr. 38(4):1257–62.

20. Sirh S, Park HR. Optimal surgical timing of aspiration for spontaneous supratentorial intracerebral hemorrhage. J Cerebrovasc Endovasc Neurosurg. 2018; Jun. 20(2):96–105.

21. Wu TY, Sharma G, Strbian D, Putaala J, Desmond PM, Tatlisumak T, et al. Natural history of perihematomal edema and impact on outcome after intracerebral hemorrhage. Stroke. 2017; Apr. 48(4):873–9.

22. Xia Z, Wu X, Li J, Liu Z, Chen F, Zhang L, et al. Minimally invasive surgery is superior to conventional craniotomy in patients with spontaneous supratentorial intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg. 2018; Jul. 115:266–73.

23. Yao Z, Hu X, You C, He M. Effect and feasibility of endoscopic surgery in spontaneous intracerebral hemorrhage: a systematic review and meta-analysis. World Neurosurg. 2018; May. 113:348–56.e2.

24. Zhou X, Chen J, Li Q, Ren G, Yao G, Liu M, et al. Minimally invasive surgery for spontaneous supratentorial intracerebral hemorrhage: a meta-analysis of randomized controlled trials. Stroke. 2012; Nov. 43(11):2923–30.
Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download