This article has been
cited by other articles in ScienceCentral.
Abstract
Objective
Pseudoaneurysms (PSAs) of the internal carotid artery (ICA) and vertebral artery are rare entities but with varied treatment options. PSAs can be spontaneous or secondary to trauma, infections, malignancies or iatrogenic. To find out the efficacy of various endovascular interventions in the management of ICA and vertebral PSAs.
Methods
The study included 14 patients diagnosed with intracranial PSAs who underwent endovascular interventions in SMS Medical College, Jaipur (Rajasthan) between June 2015 to January 2019. The clinical and radiological findings (computed tomography angiography and digital subtraction angiography) were reviewed and the consequent endovascular intervention carried out and their results were analyzed.
Results
Total 14 patients were studied out of which 8 (57.1%) were anterior circulation PSAs and 6 (42.9%) were posterior circulation PSAs There were 10 (71.4%) females and 4 (28.5%) males between the age of 9 to 65 years. Only 2 patients with PSA had past history of trauma. Coiling was done in 8 patients (57.1%), stenting in 2 patients (14.2%), parent artery occlusion in 1 patient (7.1%), glue embolization in 1 patient (7.1%) while coiling with glue in 1 patient (7.1%) and flow diverter in 2 patients (14.2%). Immediate and complete occlusion was achieved in 11 (78.6%) patients while 3 (21.4%) patients had subtotal occlusion. 11 patients under follow up till June 2019 did not report recurrence or new neurological deficit.
Conclusions
Endovascular interventions is minimally invasive and safe treatment strategy for intracranial PSAs. The ultimate choice of technique depends on clinical and imaging characteristics.
Go to :

Keywords: Pseudoaneurysm, Endovascular, Coiling, Glue embolization, Traumatic
INTRODUCTION
Pseudoaneurysms (PSAs) of the internal carotid artery (ICA) and vertebral artery are uncommon lesions. PSAs can occur spontaneously or secondary to trauma, infections, iatrogenic causes or malignancies [
6]. Early diagnosis and management is essential as they may have a potentially lethal course with a very high risk of cerebral thromboembolism and rupture. Presenting symptoms depends on size and location of PSAs along with whether it is ruptured or unruptured. Commonly, these lesions can present as intracranial haemorrhage or infarction. Both surgical and endovascular techniques are available for their management. Lately, endovascular techniques have been proven to have edge over conventional surgical management. It is less invasive and is helpful especially when surgery is contraindicated due to various reasons. Vascular surgeries have a risk of intraoperative fatal haemorrhage and/or major vessel occlusion [
3]. There are neither randomised trial data nor specific guidelines regarding the management of ICA and vertebral PSAs. We describe our experience in the endovascular treatment of ICA and vertebral PSAs secondary to various etiological factors. We also present the management algorithm for ICA PSAs followed at our institute.
Go to :

MATERIALS AND METHODS
This is an observational study conducted in the department of Neurosurgery, SMS Medical College, Jaipur (Rajasthan) from June 2015 to January 2019. All the patients were followed till June 2019. Pertinent clinical details, digital subtraction angiography (DSA), computed tomography angiography (CTA) data, mode of endovascular intervention and other relevant details were compiled for individual patient after approval from the institutional ethics committee. A total of 14 patients with ICA/vertebral PSAs were identified.
Endovascular techniques
The endovascular techniques chosen were individualized depending upon the anatomy, size and location of the lesion, cross circulation and course of parent artery. But due to lack of universal medical insurance, the financial status of patients also influenced the chosen intervention. Parent artery occlusion was done after testing for cross circulation by balloon occlusion test [
3,
4]. Patients were loaded with dual antiplatelets who underwent stenting. Dual antiplatelets were given for one month followed by lifelong single antiplatelets therapy. Coiling was done using single or double catheter technique, depending upon the size and location of the lesion. Flow diverters were used in two patients [
2,
5]. Following the endovascular treatment check DSA was done in all the cases to evaluate the effectiveness of procedure and patency of the distal circulation.
Outcome and follow up
Patients were followed up routinely and evaluated clinically. Magnetic resonance (MR) angiography/ CTA were done on follow up.
Go to :

RESULTS
There were total 14 patients consisting of 8 (57.1%) anterior circulation PSAs and 6 (42.9%) posterior circulation PSAs. 10 of the 14 cases (71.4%) were female and only 4 (28.5%) were male with age ranging between 9 years to 65 years. Most of the PSAs were spontaneous in origin; except two cases (14.2%) who had preceding history of trauma (
Table 1).
Table 1.
Clinical presentation with location and type of pseudoaneurysm, treatment received with outcome
|
Serial number/age/sex |
Location |
Presentation |
Treatment |
Immediate outcome |
Follow up |
|
1/11/F |
A3 segment left ACA ruptured |
Headache, vomiting, ICH with mass effect |
Glue |
Complete occlusion |
No aneurysm recurrence / neurological deficit at 2 years |
|
2/33/F |
Right P1 pseudoaneurysm (25×18 mm) |
Headache with right ptosis |
Coiling |
Complete occlusion |
Lost to follow up |
|
3/65/F |
Fusiform dissecting right vertebral (V3-V4 junction) aneurysm |
SAH, IVH, infarction with loss of consciousness |
Stenting |
Subtotal occlusion |
Patent stent at 18 months |
|
4/45/M |
Right distal PCA pseudoaneurysm (16×8.8 mm) |
SAH, ICH and IVH with headache, vomiting and altered sensorium |
Coiling |
Complete occlusion |
Lost to follow up |
|
5/18/M |
Left vertebral-PICA junction aneurysm |
Headache, vomiting |
Coiling |
Complete occlusion |
No aneurysm recurrence / neurological deficit at 3 years |
|
6/50/F |
Bilobed pseudoaneurysm of right A2 segment |
SAH, ICH with altered sensorium and right sided weakness |
Coiling |
Complete occlusion |
No aneurysm recurrence / neurological deficit at 2½ years |
|
7/55/F |
Left A2 dissecting aneurysm |
SAH with headache and vomiting |
Flow diverter |
Significant contrast stasis |
No aneurysm recurrence / neurological deficit at 1 year |
|
8/9/F |
Right ICA dissecting aneurysm |
Mass effect, 3rd nerve palsy |
Parent artery occlusion with coiling |
Complete occlusion |
No neurological deficit at 1 year |
|
9/26/F |
Left ophthalmic dissecting aneurysm |
Headache, decreased vision left side |
Flow diverter |
Complete occlusion |
No aneurysm recurrence / neurological deficit at 1year |
|
10/20/M |
Right P2 pseudoaneurysm |
Headache |
Coiling+Glue |
Complete occlusion |
No aneurysm recurrence / neurological deficit at 1 year |
|
11/50/F |
Right distal ACA pseudoaneurysm |
SAH, ICH, IVH, mass effect with headache, LOC, vomiting |
Coiling |
Complete occlusion |
No aneurysm recurrence / neurological deficit at 6 months |
|
12/38/F |
Left ICA supraclinoid giant pseudoaneurysm |
SAH, IVH with h/o spontaneous fall and LOC |
Coiling+stenting |
Subtotal occlusion/Expired |
- |
|
13/40/M |
Distal ACA pseudoaneurysm |
Headache, vomiting |
Coiling |
Complete occlusion |
No aneurysm recurrence / neurological deficit at 6 months |
|
14/35/F |
Right AICA pseudoaneurysm |
Headache |
Coiling |
Complete occlusion |
Right 7th nerve palsy |

The most common presenting symptom was headache (n=10). Other common complaints included altered sensorium/loss of consciousness (n=6), vomiting (n=4), hemiparesis (n=1), impaired vision (n=2), ptosis (n=1), spontaneous fall (n=1) and third nerve palsy (n=1). CT scan finding were suggestive of Subarachnoid haemorrhage (SAH) [n=6], intracerebral haemorrhage (ICH) [n=4], mass effect (n=3), intraventricular haemorrhage (n=4) and infarction (n=1).
A variety of endovascular techniques were employed for management of these lesions. Only coiling was done in 8 patients (57.1%) (
Fig. 1), stenting in 2 patients (14.2%) out of which one was stent assisted coiling, parent artery occlusion in 1 patient (7.1%), only glue in a single patient (7.1%) (
Fig. 2) while coiling with glue in 1 patient (7.1%) and flow diverter in 2 patient (14.2%) (
Fig. 3). There was no major procedure related complication except in two patients. One patient expired who had partial occlusion with contrast leakage following stent assisted coiling and other one had 7th nerve palsy secondary to coil migration. Out of total of 14 patients, 11 were under regular follow up (till June 2019) and none of them had experienced recurrence or neurological deficit.
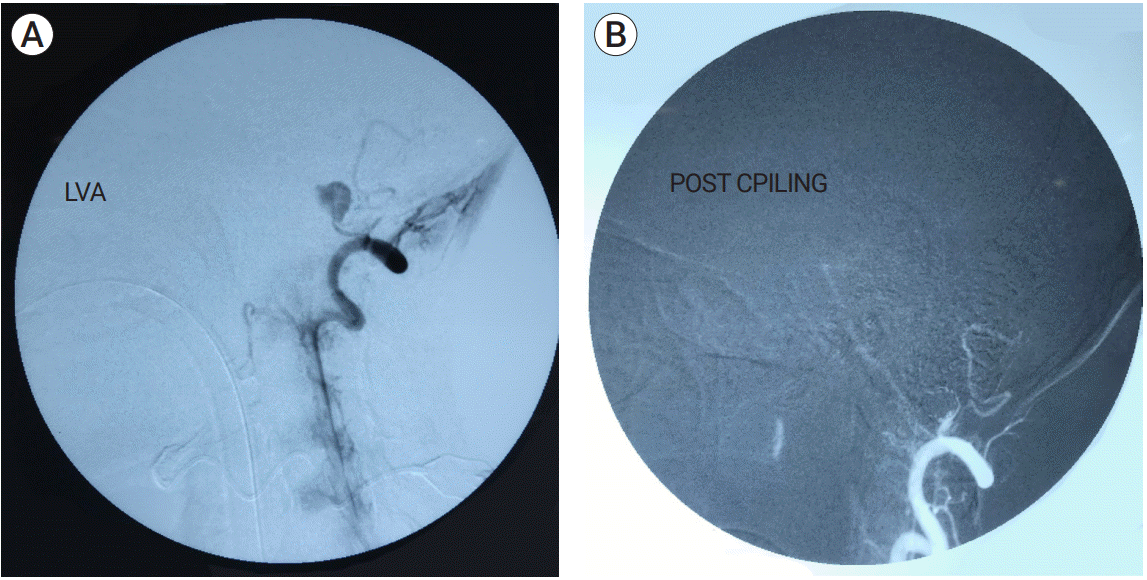 | Fig. 1.(A) Left vertebral artery pseudoaneurysm. (B) Left vertebral artery angiogram, post coiling. 
|
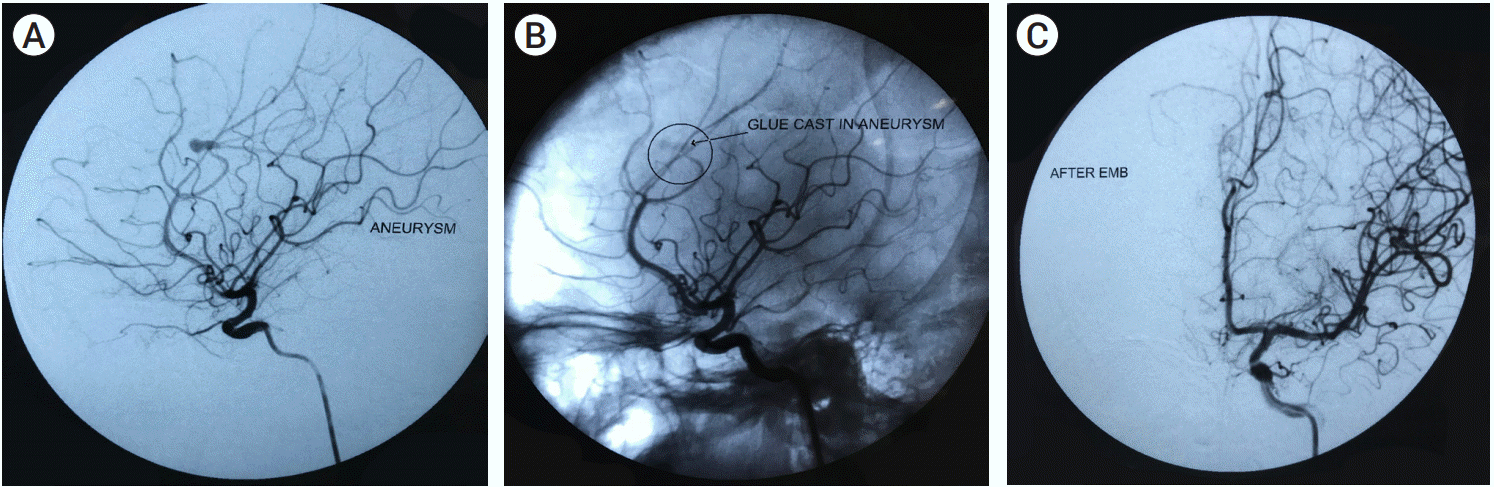 | Fig. 2.(A) Angiogram showing Distal anterior cerebral artery pseudoaneurysm. (B) Pseudoaneurysm with glue cast. (C) Post embolization DSA. DSA, digital subtraction angiography. 
|
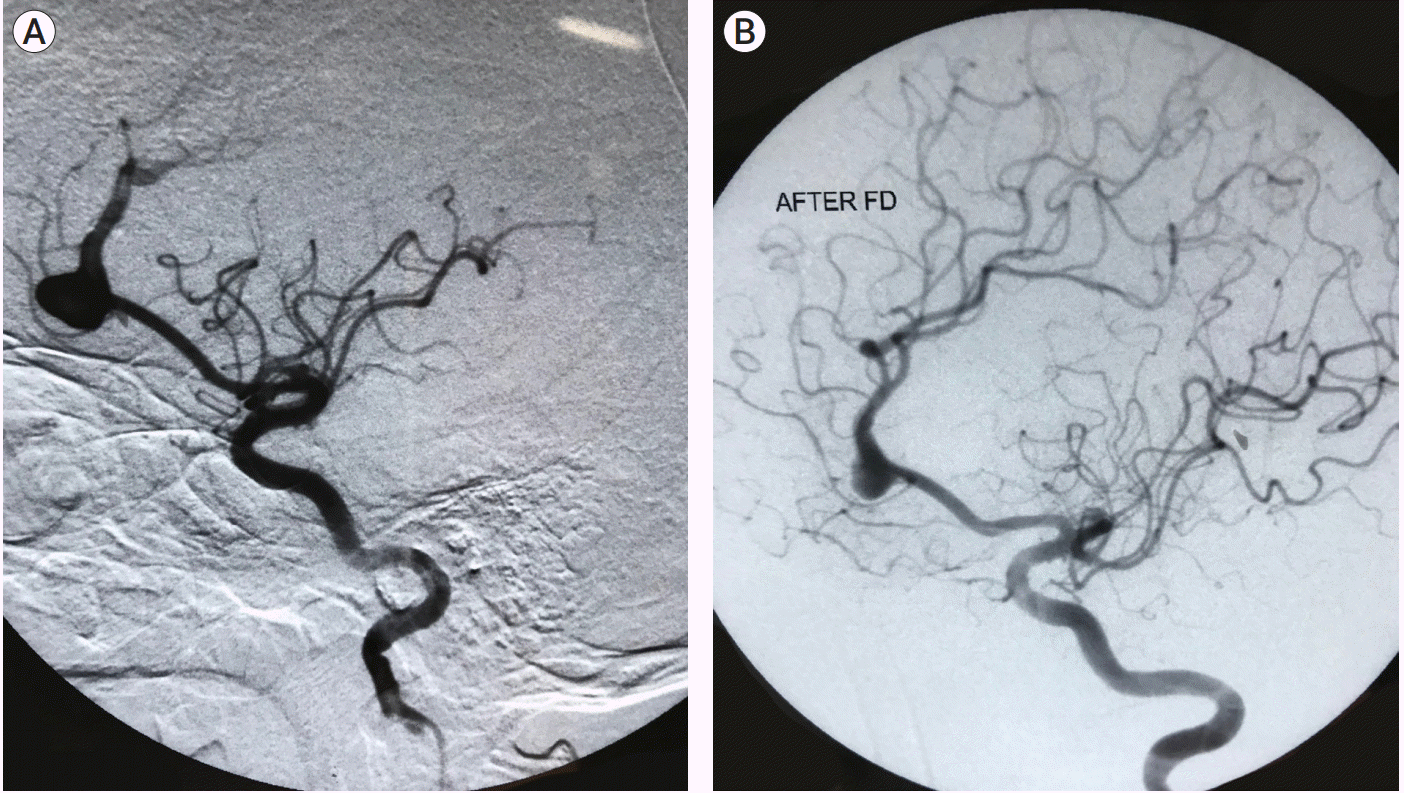 | Fig. 3.(A) Pseudoaneurysm in anterior cerebral artery. (B) Angiography post flow diverter insertion. 
|
Go to :

DISCUSSION
The main goals for the treatment of intracranial PSAs are to prevent PSA rupture, embolic events and procedure related neurological deficit. In past PSAs were treated mainly by micro- neurosurgery while nowadays both surgical and endovascular management options are available. With improvement in endovascular skills and availability of newer technology, the endovascular techniques have become the treatment of choice. Depending upon the status of collateral circulation and site of PSA both circulation preserving and parent artery occlusion technique can be used for management of intracranial PSAs. Circulation preserving techniques include coiling of PSA, stenting, stent assisted coiling, balloon assisted coiling and flow diverter placement [
2,
5,
7].
Though it is relatively easy to place stents in extracranial PSAs owing to the straighter course of artery with no side branches, the arteries giving rise to intracranial PSAs are more tortuous giving out branches to vital neurological structures [
3]. The unavailability of soft intracranial stent grafts along with the anatomical factors makes coiling and parent artery occlusion preferred treatment option in intracranial PSAs.
In our series total 10 patients underwent coiling out of which one was stent assisted coiling and one was coiling with glue. Only one patient out of the ten had favourable anatomy for stenting and managed by intracranial stent placement. Parent artery occlusion with trapping may be performed in the patients with adequate collateralisation. In our series, adequate collateral circulation was evident in a single patient who was successfully managed with parent artery occlusion with trapping without incurring new neurological deficit [
1,
4].
Limitations
Even though our study showed consistent results with cure rates and complications similar to that reported in literature, there were few shortcomings in our study. The most important limitation was small sample size with short follow up duration. Another decisive factor was the financial status of patients and its influence on the chosen modality of treatment.
Go to :

CONCLUSIONS
Although infrequent, intracranial pseudoaneurysms pose significant therapeutic challenges and are associated with considerable morbidity and mortality. Endovascular techniques have become the standard of care with few limitations.
Go to :





 PDF
PDF Citation
Citation Print
Print






 XML Download
XML Download