INTRODUCTION
Replication-defective retroviral (RDR) vectors have insufficient gene transfer efficacy and carry the risk of insertional mutagenesis. To improve the low-level transduction efficiency of these vectors, a replication-competent retroviral (RCR) vector system based on the full-length molecular clones of Moloney murine leukemia virus (MoMLV) was developed (1, 2, 3, 4, 5, 6, 7). RCR vectors have demonstrated improved efficacy of gene delivery, however, they still present the risk of accidental spread. To mitigate this risk, semi-replication competent retroviral (s-RCR) vectors were developed (8, 9). s-RCR vectors are based on two transcomplementing RDR vectors (gag-pol and env vector). They propagate transgenes as efficiently as RCR vectors and have an insert capacity of up to 7.3 kb of transgene, compared to 1.3 kb for RCR vectors. The higher capacity of s-RCR vectors allows for the insertion of additional therapeutic transgenes.
Fusogenic membrane glycoproteins (FMGs) are viral envelope proteins which induce tumor cell killing through syncytium formation (10, 11, 12, 13). In previous studies, the cytotoxicity of FMGs from human immunodeficiency virus (HIV), human endogenous retrovirus-W (HERV-W), vesicular stomatitis virus G glycoprotein (VSV-G), F and H genes of measles virus, feline endogenous virus RD-114, and gibbon ape leukemia virus (GALV) were evaluated (14, 15, 16). It is known that the cytotoxic activity of the R peptide truncated GALV envelope glycoprotein (GALV FMG) is higher than that of any other FMG (11, 15). FMG-mediated syncytium formation requires the presence of relevant cell surface receptor, and the induction of syncytia by some MLVs depend on receptor density (17). In addition, the bystander effect of FMG-mediated tumor cell killing is one log higher than that of suicide genes such as herpes simplex virus-1 thymidine kinase/ (HSV-1tk)/ Ganciclovir (GCV) or cytosine deaminase (CD)/5-fluorocytosine (5-FC) (10, 18).
Trajcevski et al. reported that s-RCR vectors pseudotyped with amphotropic MLV 4070A envelope allow propagations as efficient as those of RCR vector. In studies using MLV-sensitive mice, the s-RCR vector did not spread to other sites and showed no leukemia (9). Other researchers have successfully constructed s-RCR vectors pseudotyped with VSV-G (8). They incorporated VSV-G into the env vector to see if it could serve both as a pseudotyping envelope and a cytotoxic protein. s-RCR vectors pseudotyped with VSV-G induced both viral spread and progressive syncytial formation. However, VSV-G protein caused membrane fusion only at low pH (19, 20, 21). Therefore, in this study, we have attempted to use GALV FMG, which causes membrane fusion at neutral pH.
GALV FMG is a C-terminally truncated form of GALV envelope glycoprotein, from which the 16 amino acid R peptides have been deleted. The R peptides are cleaved by viral proteases during virion maturation, inhibiting the fusion of the envelope until the virus leaves the cell. Previous studies have reported that MoMLV lacking R peptide could induce cell-to-cell fusion in NIH3T3 cells (22). MoMLV does not induce cell-to-cell fusion in human cells, but GALV FMG is highly fusogenic to receptor expressing human cells. GALV infects human cells through the Pit1 (SLC20A1) mammalian type III inorganic phosphate transporter, which is ubiquitously expressed on human cancer cells (23, 24, 25).
To take advantage of the therapeutic potential of GALV FMG, we developed the s-RCR vector system in which the gag-pol and GALV FMG genes are split into two vectors. We showed that during viral spread, s-RCR vectors induced cancer cell killing by virtue of the cytotoxicity of GALV FMG. Our data demonstrate that the s-RCR vectors encoding GALV FMG could play an important role in cancer gene therapy.
Go to : 
MATERIALS AND METHODS
Cell lines
The 293T human embryonic kidney cells (ATCC CRL-11268), PG13/LNc8 cells (ATCC CRL-10685), HT1080 human fibrosarcoma cells (ATCC CCL-121), and TE671 human rhabdomyosarcoma cells (ATCC CRL-8805) were maintained in Dulbecco’s modified Eagle medium (DMEM) supplemented with 10% fetal calf serum (FCS), 100 U/mL penicillin, and 100 μg/mL streptomycin.
Construction of s-RCR vectors
To obtain GALV env and GALV FMG, total DNA was extracted from PG13/LNc8 cells with G-spin™ Total DNA Extraction Mini Kit (iNtRON biotechnology, Seoul, Korea) according to the manufacturer’s instructions. The sequences of primers are shown below: GALV F: 5’-AGATCTGCCACCATGGTATTGCTGCCTGGGTCCATG-3’ (BglII restriction site is underlined), GALV R: 5’-CTCGAGTTAAAGGTTACCTTCGTTCTCTAGG-3’ (XhoI restriction site is underlined), FMG F: 5’-AGATCTGCCACCATGGT ATTGCTGCCTGGGTCCATG-3’ (BglII restriction site is underlined), FMG R: 5’-CTCGAGTTACAGAATTTTAACTGCACTTATCCT’ (XhoI restriction site is underlined). Polymerase chain reaction (PCR) products of the GALV env and GALV FMG were ligated into the pGEM®-T Easy Vector System (Promega, Madison, WI, USA). pGEM-T-GALV env and pGEM-T-GALV FMG were digested with the restriction enzymes EcoRI and XhoI; the digested fragments were ligated into previously constructed pCLXSN-IRES-EGFP to generate pCLXSN-GALV-IRES-EGFP and pCLXSN-GALV FMG-IRES-EGFP. To create MoMLV-gag-pol-IRES-EGFP, previously developed RCR vector (MoMLV-10A1-EGFP) was digested with restriction enzyme EcoRI, and then self-ligated to delete env gene. Construction of plasmids MoMLV-VSV-G and MoMLV-EGFP has been described previously (26). To determine the viral titers, GFP-positive cells were analyzed by FACSCaliburTM flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ USA).
s-RCR virus production
To generate s-RCR vectors encoding GALV FMG, 293T cells were transiently co-transfected with MoMLV-gag-pol- IRES-EGFP, alongside either pCLXSN-GALV-IRES-EGFP or pCLXSN-GALV FMG-IRES-EGFP, using the CalPhos Mammalian Transfection Kit (TaKaRa Bio, Shiga, Japan). To investigate whether the cytotoxicity of GALV FMG could be enhanced by the addition of VSV-G, pCLXSN-GALV FMG-IRES-EGFP and MoMLV-VSV-G were co-transfected to generate VSV-G pseudotyped s-RCR vectors. One milliliter aliquots of viral supernatants at 6 days post-transfection of 293T cells were added to TE671 and HT1080 cells in the presence of 8 µg/ml polybrene.
Construction of gag-pol vector encoding GALV FMG
To check if the degree of syncytium formation is dependent on whether GALV FMG is located on the gag-pol or the env vector, MoMLV-gag-pol-IRES-GALV FMG was constructed by replacing EGFP of MoMLV-gag-pol-IRES-EGFP with GALV FMG. To produce gag-pol vector containing GALV FMG, MoMLV-gag-pol-IRES-GALV FMG and MoMLV-EGFP were co-transfected into 293T cells. For the production of env vector containing GALV FMG, pCLXSN-GALV FMG-IRES-EGFP and MoMLV-gag-pol-IRES-EGFP were co-transfected into 293T cells. To determine the degree of syncytium formation, HT1080 cells were infected with 1 ml of supernatants collected at 6 days post-transfection. The syncytium formation by GALV FMG was observed using fluorescence microscopy. The number of nuclei per syncytium counted from three random fields per well by microscopic examination. Results were reported as the average of two independent experiments.
Go to : 
RESULTS
Generation of the s-RCR vector system
To produce gag-pol vector carrying EGFP reporter transgene, MoMLV-gag-pol-IRES-EGFP was constructed from replication- competent MoMLV-10A1-IRES-EGFP recombinant vector by deleting 10A1 env gene (Fig. 1). To create the env vector (pCLXSN-GALV FMG-IRES-EGFP), we inserted GALV FMG into retroviral vector pCLXSN, with an IRES-EFGP positioned directly downstream of GALV FMG. To obtain high titers of viral stocks, previously constructed MoMLV-VSV-G was used to produce s-RCR vectors. MoMLV-VSV-G was generated by positioning VSV-G directly downstream of the gag-pol of MoMLV-gag-pol-IRES-EGFP (26). To test whether gag-pol could increase syncytium formation of GALV FMG, all-in-one MoMLV-gag-pol-IRES-GALV FMG was also constructed.
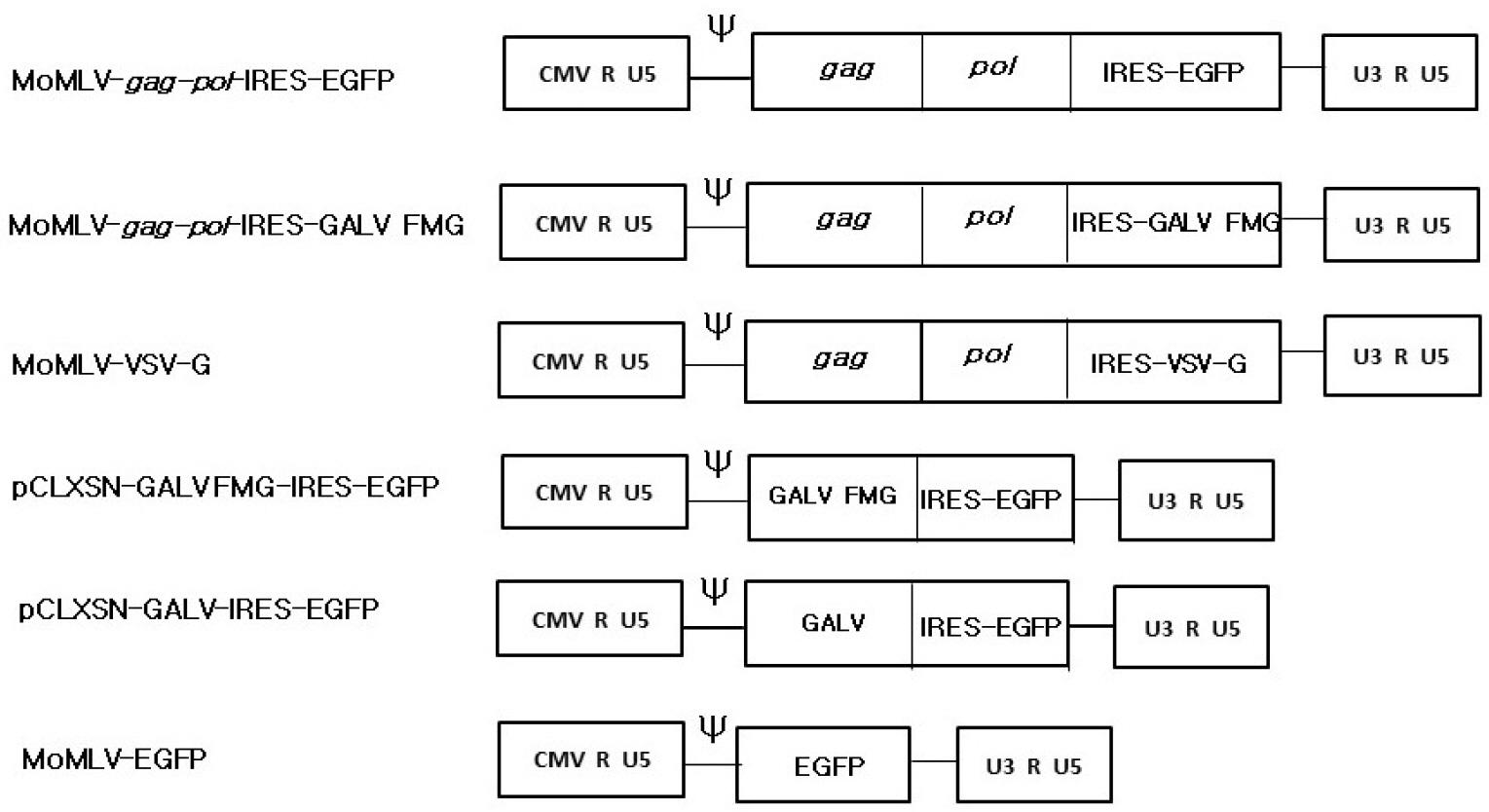 | Fig. 1Schematic structure of semi-replication-competent retroviral vectors. All vectors were derived from the full-length molecular clone of MoMLV. The MoMLV-gag-pol-IRES-EGFP vector contains an IRES-EGFP cassette between the pol gene and the 3' UTR. The MoMLV-gag-pol-IRES-GALV FMG is a replication-defective vector that contains the 16-amino acid R-peptide-truncated GALV env (GALV FMG). For the MoMLV-VSV-G, EGFP of the MoMLV-gag-pol-IRES-EGFP was replaced with the fusogenic envelope G glycoprotein of the vesicular stomatitis virus (VSV-G). The pCLXSN-GALV FMG-IRES-EGFP was generated by cloning GALV FMG into pCLXSN retroviral vector. pCLXSN-GALV-IRES-EGFP was generated by replacing GALV FMG of pCLXSN-GALV FMG-IRES-EGFP with GALV env. In MoMLV-EGFP, EGFP was inserted into pCLXSN retroviral vector. |
Production of s-RCR virus
To produce s-RCR viruses, MoMLV-gag-pol-IRES-EGFP with either pCLXSN-GALV FMG-IRES-EGFP or pCLXSN-GALV-IRES-EGFP was co-transfected into 293T cells. We observed a progressive increase, both in the percentage of EGFP-positive cells and as well as syncytium formation, at 2 days post-transfection. The viral titers from 293T cells transiently transfected with MoMLV-gag-pol-IRES-EGFP and pCLXSN-GALV FMG-IRES-EGFP at 2 days post-transfection were 3ⅹ106 transducing unit (TU)/ml. These titers were a little higher than those obtained with MoMLV-gag-pol-IRES-EGFP and pCLXSN-GALV-IRES-EGFP (2×106 TU/ml). This result is consistent with previous reports that truncation of the GALV R peptide increases the incorporation of envelope protein into infectious viral particles (27, 28).
s-RCR from MoMLV-gag-pol-IRES-EGFP and pCLXSN-GALV-IRES-EGFP showed reduced viral spread upon passage of the transfected cells (Fig. 2). In contrast, s-RCR from MoMLV-gag-pol-IRES-EGFP and pCLXSN-GALV FMG-IRES-EGFP showed subsequent EGFP propagation and progressive syncytium formation. When flow cytometry analysis of EGFP expression was performed at 11 days post-transfection, cells transfected with GALV FMG showed almost 80% EGFP-positive, while cells transfected with GALV env showed about 30% EGFP-positive (Fig. 3).
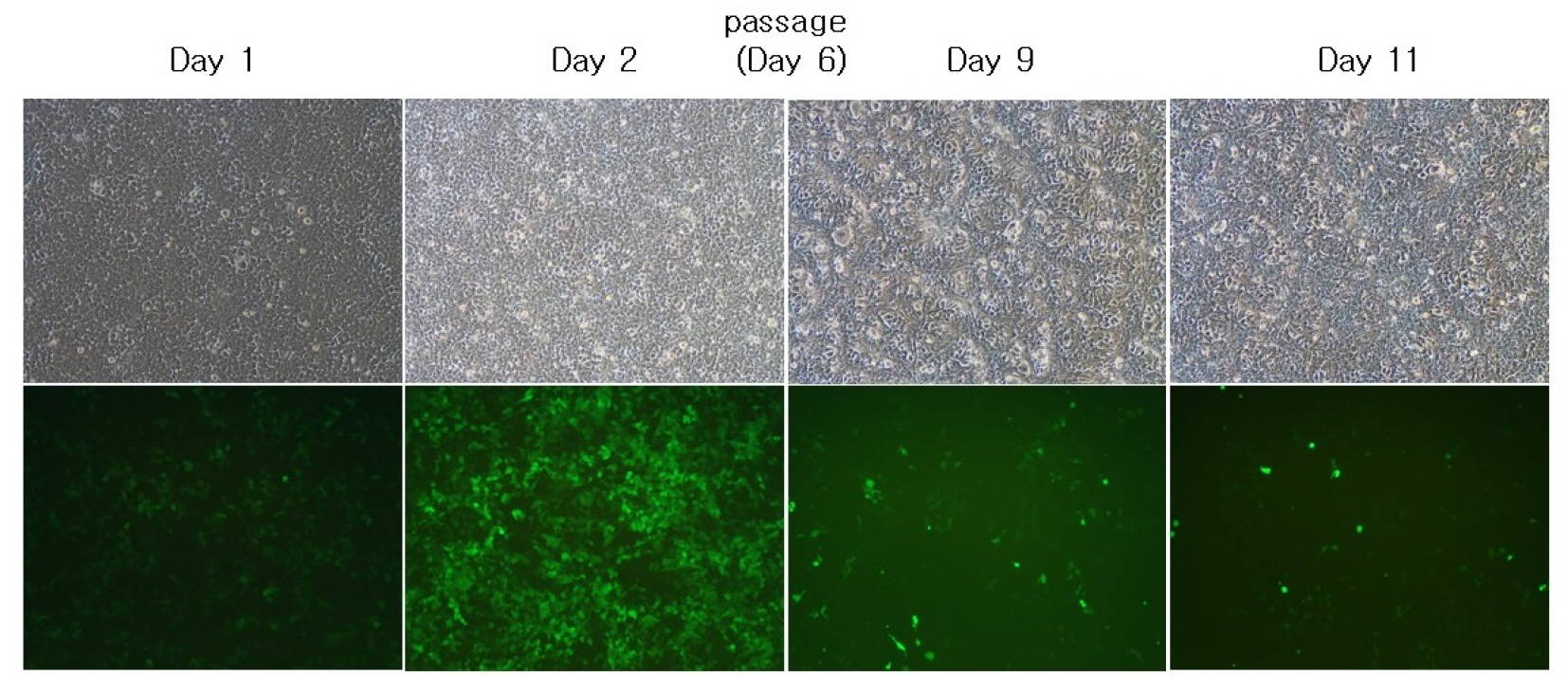 | Fig. 2In vitro EGFP gene propagation by semi-replication-competent retroviral vectors. 293T cells were co-transfected with MoMLV-gag-pol-IRES-EGFP and pCLXSN-GALV-IRES-EGFP. Cells were passaged on day 6 when they became confluent. At each different time-point (Day 1, 2, 9 and 11), pictures were taken. Syncytium formation was observed using phase contrast microscopy (top panel) or fluorescence microscopy. Objective magnification, X100. |
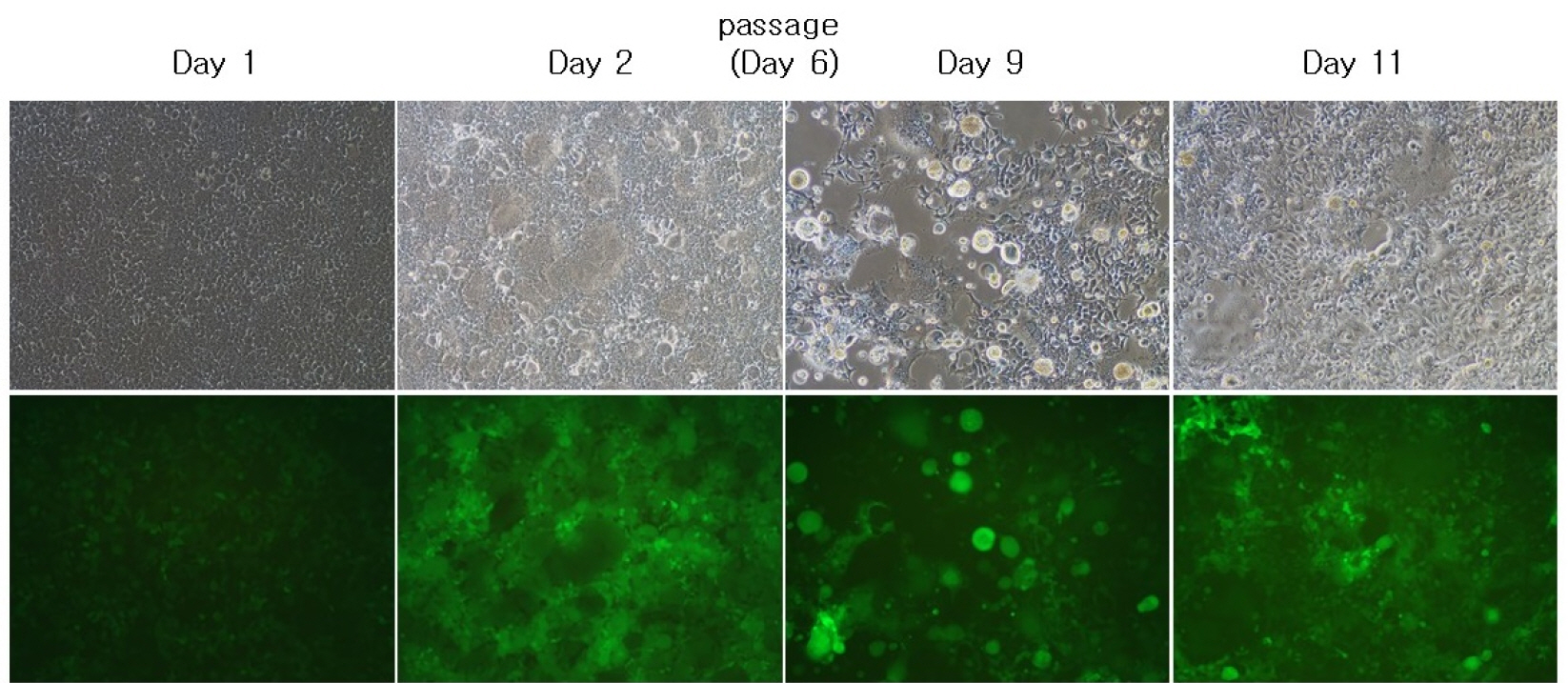 | Fig. 3In vitro EGFP gene propagation by semi-replication-competent retroviral vectors. 293T cells were co-transfected with MoMLV-gag-pol-IRES-EGFP and pCLXSN-GALV FMG-IRES-EGFP. Cells were passaged on day 6 when they became confluent. At each different time-point (Day 1, 2, 9 and 11), pictures were taken. Syncytium formation was observed using phase contrast microscopy (top panel) or fluorescence microscopy. Objective magnification, X100. |
Construction of VSV-G pseudotyped s-RCR vector for cancer gene therapy
To obtain the synergistic effect of cytotoxicity due to co-expression of VSV-G and GALV FMG, we incorporated the VSV-G into gag-pol vector. When pCLXSN-GALV FMG-IRES-EGFP alone was transfected into 293T cells, limited syncytium formation was observed at 2-6 days (Fig. 4A). In contrast, when MoMLV-VSV-G + pCLXSN-GALV FMG-IRES-EGFP were co-transfected into 293T cells, extensive syncytium formation and subsequent cell death were observed at 9 days post-transfection (Fig. 4B). Although, progressively developing toxicity was also observed in cells transfected with MoMLV-VSV-G alone (Fig. 4C), co-expression of VSV-G and GALV FMG induced extensive syncytium formation.
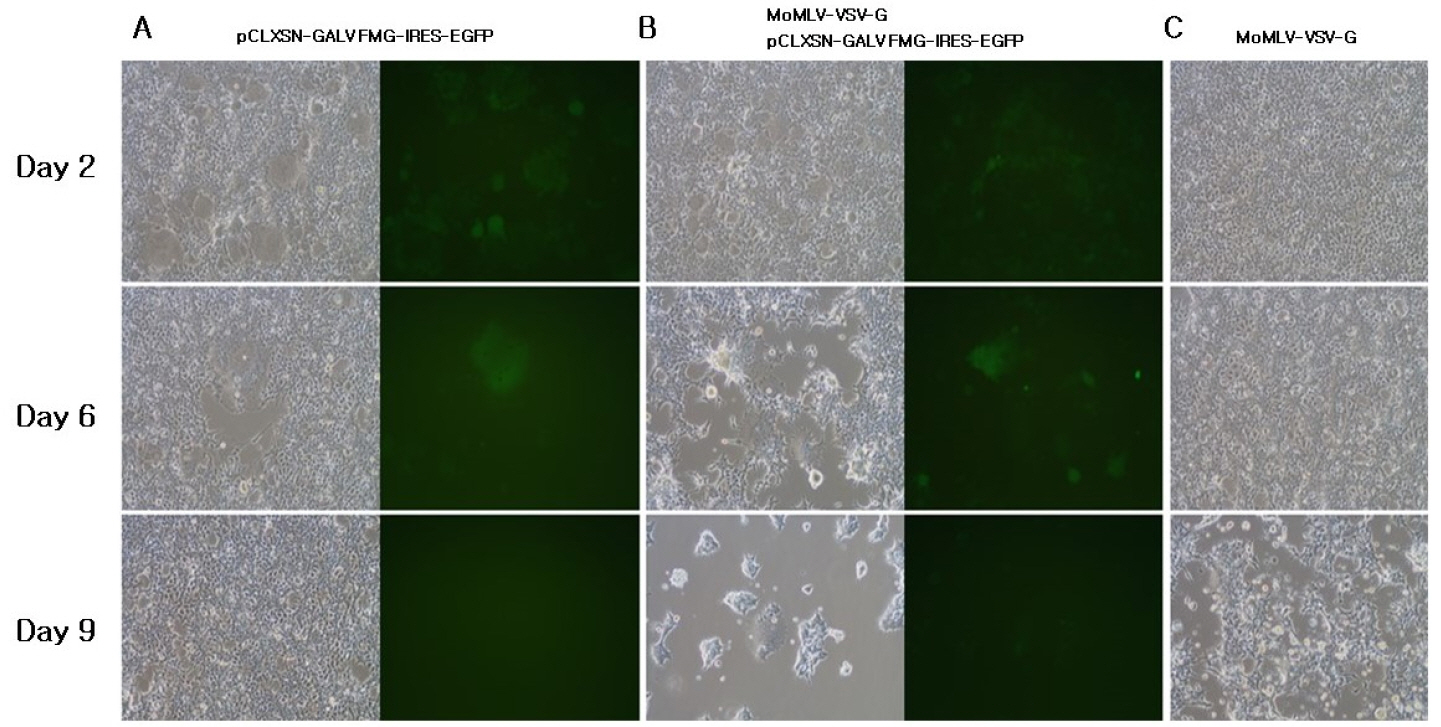 | Fig. 4Characterization of VSV-G pseudotyped s-RCR vector. 293T cells were transfected with pCLXSN-GALV FMG-IRES-EGFP (A), MoMLV-VSV-G + pCLXSN-GALV FMG-IRES-EGFP (B), MoMLV-VSV-G (C). At each different time-point (Day 2, 6 and 9), syncytium formation was observed using phase contrast microscopy or fluorescence microscopy. Objective magnification, X100. |
When TE671 and HT1080 cells were infected with the s-RCR viruses collected from MoMLV-VSV-G + pCLXSN-GALV FMG-IRES-EGFP co-transfected 293T cells at an MOI of 5, extensive syncytium formation was observed in HT1080 cells at 3 days post-transfection (Fig. 5B). In contrast, syncytium formation occurred to a lesser extent in TE671 compared to HT1080 cells (Fig. 5A). The number of nuclei per syncytia was an average of >50 in the HT1080 cells, while an average of ± 20 nuclei per syncytia were detected in the TE671 cells.
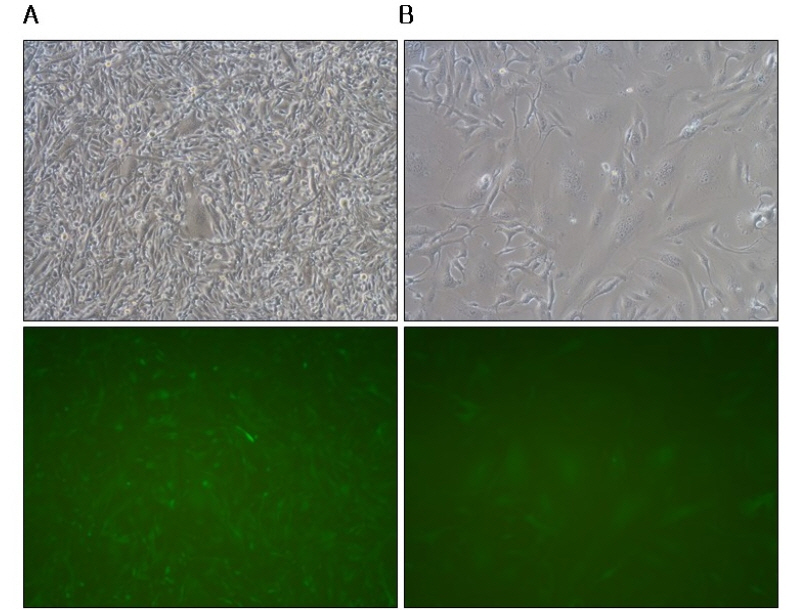 | Fig. 5Syncytium formation on human cancer cells by VSV-G pseudotyped s-RCR vector. (A) TE671 cells and HT1080 cells (B) were infected with the supernatants collected from MoMLV-VSV-G + pCLXSN-GALV FMG-IRES-EGFP co-transfected 293T cells at an MOI of 5. Syncytium formation was observed at 72 h after infection using phase contrast microscopy (top panel) or fluorescence microscopy. Objective magnification, X200. Number of nuclei per syncytia counted from three independent experiments. |
Co-expression of GALV FMG in gag-pol vector enhance syncytium formation
The s-RCR vector system is based on gag-pol and env vectors. Previous studies have suggested that gag-pol vector spreads faster than env vector in the s-RCR vector system (9). To test whether gag-pol vector containing GALV FMG enhances syncytium formation, we constructed all-in-one MoMLV-gag-pol-IRES-GALV FMG. When 293T cells were transfected with either MoMLV-gag-pol-IRES-GALV FMG + MoMLV-EGFP or MoMLV-gag-pol-IRES-EGFP + pCLXSN-GALV FMG-IRES-EGFP, stronger syncytium formation was achieved in gag-pol vectors than in env vectors. To confirm that s-RCR viruses were released from transfected cells, these viruses were used to infect HT1080 (Fig. 6) at an MOI of 5. Although s-RCR viruses from MoMLV-gag-pol-IRES-EGFP + pCLXSN-GALV FMG-IRES-EGFP also induced syncytium formation, it was more extensive in s-RCR viruses from MoMLV-gag-pol-IRES-GALV FMG + MoMLV-EGFP. MoMLV-gag-pol-IRES-GALV FMG inducted an average of ± 40 nuclei, but pCLXSN-GALV FMG-IRES-EGFP-mediated syncytia contained an average of ± 25 nuclei.
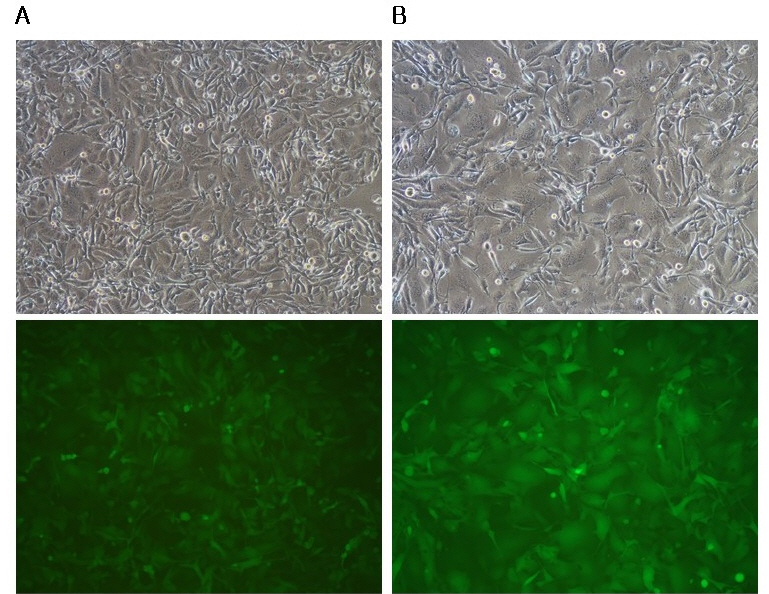 | Fig. 6Expression of GALV FMG in the gag-pol vector enhance syncytium formation. HT1080 cells were infected with the supernatants collected from MoMLV-gag-pol-IRES-EGFP + pCLXSN-GALV FMG-IRES-EGFP (A) and MoMLV-gag-pol-IRES-GALV FMG + MoMLV-EGFP (B) co-transfected 293T cells at an MOI of 5. Syncytium formation was observed at 72 h after infection using phase contrast microscopy (top panel) or fluorescence microscopy. Objective magnification, X100. Number of nuclei per syncytia counted from three independent experiments. |
Go to : 
DISCUSSION
Previous studies have reported the development of a s-RCR vector system using two complementary RDR vectors (8, 9). These vectors are safer than RCR vectors and more efficient in transgene propagation compared to RDR vectors. Coupled with their higher insert capacity, which allows for the insertion of large therapeutic transgenes, they have distinct advantages over the RCR vectors, the latter being unstable at insert sizes of 1.6 kb or more (29, 30). In this study, s-RCR vectors derived from GALV FMG were developed for cancer gene therapy (Fig. 1).
As shown in Figs. 2 and 3, an increased proportion of EGFP-positive cells is observed after s-RCR vector transfection. Interestingly, when transfected cells were passaged at day 6, the percentage of pCLXSN-GALV FMG-IRES-EGFP-transduced cells remained stable (almost 80% of GFP positive), whereas the percentage of pCLXSN-GALV-IRES-EGFP-transduced cells was reduced (30% of GFP positive). These interesting results suggest that syncytium can enhance cell-to-cell spread of s-RCR vector encoding GALV FMG. Previous studies have also reported that the expression of FMG in cancer cells could enhance the therapeutic efficacy of an oncolytic adenovirus and herpes simplex virus (12, 13). However, since cell-to-cell spread can occur in a variety of ways (31, 32), further studies are needed to reveal the exact mechanism of the cell-to-cell spread of s-RCR produced from MoMLV-gag-pol-IRES-EGFP + pCLXSN-GALV FMG-IRES-EGFP transfected 293T cells.
Generally, VSV-G is used to make retroviral pseudotype since it can induce efficient transduction of several cell types (33). In addition, retroviruses pseudotyped with VSV-G can be concentrated by ultracentrifugation. In this study, whether VSV-G acts as a pseudotyping envelope and can show synergistic cytotoxicity with GALV FMG, previously constructed MoMLV-VSV-G was used to produce of s-RCR vectors. As shown in Fig. 4B, co-transfection of MoMLV-VSV-G + pCLXSN-GALV FMG-IRES-EGFP induced massive syncytium formation at day 9. As expected, cytotoxicity due to GALV FMG and VSV-G was stronger than that of VSV-G alone (Fig. 4C). Although both GALV FMG and VSV-G can act as pseudotyping envelopes, the latter seems to increase the production of infectious s-RCR viruses. This is because VSV-G has been reported to produce a higher titer of pseudotype retroviruses than other retroviral envelope proteins. This result agrees with a previous study which reported that the co-expression of GALV FMG and VSV-G avoids envelope mixing (18). As shown in Fig. 5, s-RCR viruses collected from transfected 293T cells induced massive syncytium formation in HT1080 compared to those from TE671. These results suggest that HT1080 cells express more receptors for GALV than TE671. In addition, since VSV-G is known to require acid shock to induce fusion in HT1080, it is believed that the syncytium formation shown in Fig. 5 is due to GALV FMG.
Previous studies have shown that gag-pol could increase syncytium formation in truncated ecotropic envelope-expressing NIH3T3 cells (22). We also evaluated whether the presence of GALV FMG in the gag-pol vector could affect the degree of syncytium formation. s-RCR viruses produced from all-in-one MoMLV-gag-pol-IRES-GALV FMG + MoMLV-EGFP induced extensive syncytium formation, whereas a much lower level of syncytium formation was observed when GALV FMG was present in env vector (Fig. 6). It is believed that this occurs because the degree of virus spread from all-in-one MoMLV-gag-pol-IRES-GALV FMG + MoMLV-EGFP is faster than that from MoMLV-gag-pol-IRES-EGFP + pCLXSN-GALV FMG-IRES-EGFP vector. Indeed, pCLXSN-GALV FMG-IRES-EGFP vector-transduced cells blocks re-infection by gag-pol vector using the same envelope. This interference phenomenon probably led to lower level of syncytium formation. Additionally, our results suggest that the all-in-one MoMLV-gag-pol-IRES-GALV FMG vector can act as a replication- competent vector, just like the previously constructed all-in-one MoMLV-VSV-G (26).
In this study, s-RCR vectors encoding GALV FMG were constructed, which induced syncytium formation due to the cytotoxicity of GALV FMG. In addition, we inserted GALV FMG or the fusogenic envelope G glycoprotein of the VSV into gag-pol vector to improve the cytotoxicity of s-RCR vector system. These novel s-RCR vectors significantly enhanced syncytium formation in transfected as well as infected cancer cell lines. Hence, these s-RCR vector systems are useful tools for cancer treatment.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download