INTRODUCTION
Burkholderia cepacia is a member of B. cepacia complex (BCC) that is a group of catalase-positive, lactose non-fermenting, gram-negative bacteria comprised of at least 20 different species, including B. cepacia, B. multivorans, B. cenocepacia, B. vietnamiensis, B. stabilis, B. ambifaria, B. dolosa, B. anthina, and B. pyrrocinia (1, 2). B. cenocepacia and B. multivorans are the most frequently isolated from the patients with cystic fibrosis belonging to the BCC (3, 4). B. cepacia has also emerged as an important human pathogen, especially in healthcare settings, although this microorganism was first discovered as a causative agent of onion skin rot (5, 6). BCC bacteria, including B. cepacia, pose little medical risk to healthy people, but the patients with underlying diseases such as chronic granulomatous diseases, chronic lung diseases or hematologic malignancies are susceptible to BCC infection (4, 7). BCC exhibits a relatively low virulence, but produces several virulence-associated determinants, including elastases, gelatinases, hemolysins, lipases, proteases, and siderophores, have been determined, which may play a role in colonization and infection in the hosts and survival of bacteria in environment (8, 9, 10). However, secretomes associated with bacterial pathogenesis have not been fully characterized.
All gram-negative bacteria produce and secrete outer membrane vesicles (OMVs) sized with 20-300 nm (11). OMVs are a secreted cargo that transports toxins, virulence factors, and other bacterial molecules to the host cells and can modulate physiology of host cells (12, 13). Gram-negative bacterial pathogens, including Escherichia coli (14), Pseudomonas aeruginosa (15), Acinetobacter baumannii (16), A. nosocomialis (17), Stenotrophomonas maltophilia (18), and BCC (19), secrete OMVs during in vitro culture and the secreted OMVs induce host cell cytotoxicity and pro-inflammatory responses. We recently demonstrated that B. cepacia ATCC 25416 and two clinical isolates secreted OMVs during in vitro culture (20). Moreover, B. cepacia ATCC 25416 produced more OMVs under antibiotic stress conditions such as sub-minimum inhibitory concentrations of ceftazidime, meropenem, and trimethoprim/sulfamethoxazole, than under antibiotic-free conditions. OMVs isolated from B. cepacia cultured under antibiotic stress conditions induced significantly higher pro-inflammatory responses in lung epithelial A549 cells than OMVs from B. cepacia cultured under antibiotic-free conditions. However, bacterial molecules associated with the cytotoxicity or pro-inflammatory responses in B. cepacia OMVs have not been determined. The aim of this study was to analyze the proteomes of OMVs produced by B. cepacia and investigate their contribution to the induction of host cell cytotoxicity and pro-inflammatory responses in vitro.
Go to : 
MATERIALS AND METHODS
Bacterial strain and cell culture
B. cepacia ATCC 25416 was purchased from the American Type Culture Collection (Manassas, VA, USA). Bacteria were cultured in lysogeny (LB) broth with shaking at 37°C. A549 cells originated from human lung epithelial cells were purchased from the Korean Cell Line Bank (Seoul, Korea). A549 cells were grown in RPMI 1640 medium (HyClone, Logan, UT, USA) supplemented with 10% heat-inactivated fetal bovine serum (HyClone), 2 mM L-glutamine, and 1,000 U/mL penicillin G 37°C in a humidified atmosphere with 5% CO2. Cells were seeded in 6- and 96-well plates for the cytokine gene expression and cell viability assays, respectively.
Isolation of OMVs
The OMVs of B. cepacia ATCC 25416 were purified from bacterial culture supernatants as previously described (20, 21). Bacteria were cultured to 1.5 optical density at A600 (OD600) in 1 L of LB broth with shaking at 37°C. Bacterial culture was centrifuged at 8,000 g for 20 min at 4°C and supernatants were filtered using a bottle-top filter with a 0.22 μm membrane. The filtered supernatant samples were concentrated using a QuixStand Benchtop System (GE Healthcare, Amersham, UK) with a 500 kDa hollow fiber membrane (GE Healthcare). OMVs were collected by ultracentrifugation at 150,000 g for 3 h at 4°C and then washed in phosphate-buffered saline (PBS) followed by another ultracentrifugation cycle. OMV pellets were resuspended in 100-200 μl of PBS. The protein concentration of OMVs was determined using a modified BCA assay (Thermo Scientific, Waltham, MA, USA). The purified OMVs were streaked on blood agar plates to check for sterility and then stored at -80°C until use. B. cepacia OMVs were treated with 0.1 μg/ml proteinase K (Fermentas, St. Leon-Rot, Germany) for 3 h at 50°C for the degradation of OMV proteins (22).
Protein identification
Proteins in B. cepacia OMVs were identified using one-dimensional gel electrophoresis and liquid chromatography-tandem mass spectrometry (1-DE-LC-MS/MS) as previously described (23, 24). Proteins of OMVs (15 μg) were separated on a 12% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and divided into eight fractions according to molecular weight. In-gel digestion was performed as previously described (24). Tryptic peptide mixtures were recovered after tryptic digestion, and the peptide extracts were pooled and lyophilized. Peptide samples were then concentrated on a 2G-V/V trap column (Waters, Milford, MA, USA), and concentrated peptides were eluted into a 10 cm × 75 μm (i.d.) C18 reversed-phase column at a flow rate of 300 nl/min. High performance liquid chromatography conditions and search parameters for tandem mass spectrometry (MS/MS) analysis were applied. All MS and MS/MS spectra were acquired using an LTQ-Velos ESI ion trap mass spectrometer in data-dependent mode. For protein identification, nano-LC-MS/MS spectra were searched using MASCOT version 2.4 (Matrix Science, UK) with protein sequences obtained from the B. cepacia ATCC 25416 genome. A proteomic analysis was performed in triplicate with different samples, and proteins identified in all three experiments were analyzed. Locations of proteins were predicted using the subcellular location prediction program, CELLO version 2.5 (http://cello.life.nctu.edu.tw/). The exponentially modified protein abundance index (emPAI) and mol% were acquired using MASCOT software (25). Proteins identified in B. cepacia OMVs were classified according to Gene Ontology (GO) functions using Blast2Go Pro software (https://www.blast2go.com/).
Cell viability
Cell viability was measured using the 3-[4,5-dimethylthiazol-2-yl]-2,5 diphenyltetrazolium bromide (MTT) assay (Abcam, Cambridge, UK). A549 cells were seeded at a concentration of 2.0 × 104/well in a 96-well microplate. Cells were incubated with either intact B. cepacia OMVs or proteinase K-treated OMVs for 24 h and then cell viability was measured 2 h after treatment with MTT reagent at 600 nm.
Quantitative real-time polymerase chain reaction (qPCR) of cytokine genes
A549 cells cultured in 6-well plates were incubated with 0, 10, and 20 μg/ml of intact B. cepacia OMVs or proteinase K-treated OMVs for 6 h. Total RNA was extracted using an RNeasy Mini Kit (Qiagen, Valencia, CA, USA) according to the manufacturer’s instructions. cDNA was synthesized by the reverse transcription of 2 μg of total RNA using oligo dT primers and TOPscriptTM reverse transcriptase (Enzynomics, Daejeon, Korea) in a total reaction volume of 20 μl. qPCR was performed to determine the expression levels of genes encoding glyceraldehyde 3-phosphate dehydrogenase (GAPDH), interleukin (IL) 1B, IL6, IL8, tumor necrosis factor (TNF), and C-C motif chemokine ligand 2 (CCL2) as previously described (26, 27). Quantification of the gene transcripts was performed using a StepOnePlus™ Real-Time PCR System (Applied Biosystems, Foster City, CA, USA) with TOPreal™ qPCR 2X PreMIX (SYBR Green with high ROX) (Enzynomics) according to the manufacturer’s instructions. The amplification specificity was evaluated using melting curve analysis. Gene expression was normalized to GAPDH expression in each sample, and the fold change was determined using the ΔΔCt method. Each experiment was performed in triplicate.
Data analysis and statistics
Cell death and cytokine gene expression between intact OMVs and proteinase K-treated OMVs were analyzed using Student’s t-test. Differences of P < 0.05 were considered statistically significant.
Go to : 
RESULTS
Proteomes of B. cepacia OMVs
B. cepacia ATCC 25416 was cultured in LB broth and then OMVs were isolated from the culture supernatant. A proteomic analysis of B. cepacia OMVs was performed using 1-DE-LC-MS/MS. A total of 265 proteins were identified in OMVs isolated from B. cepacia ATCC 25416 cultured in LB broth. The most abundant 30 OMV proteins analyzed by emPAI and mol% were presented in Table 1. Of the 265 proteins, 179 (67.5%), 32 (12.1%), 27 (10.2%), 17 (6.4%), and 10 (3.8%) were predicted to be located in the cytoplasm, inner membrane, periplasmic space, outer membrane, and extracellular compartment, respectively (Fig. 1A). A total of 168 proteins identified in the B. cepacia OMVs were classified into 19 groups based on GO functions; biosynthetic process-associated proteins (n = 51) were the most common category (Fig. 1B). However, 97 proteins could not be classified into a particular functional group, due to poor characterization. Several putative virulence-associated proteins, including phosphocholine-specific phospholipase C (WP_027788973.1), ATP-dependent protease HslU (WP_027788872.1), ATP-dependent zinc metalloprotease FtsH (WP_021163358.1), and ATP-dependent Clp protease (WP_027786662.1), were identified in OMVs. These results indicate that B. cepacia OMVs present a diverse protein profile comprising molecules derived from the cytoplasm, membrane, and extracellular compartment.
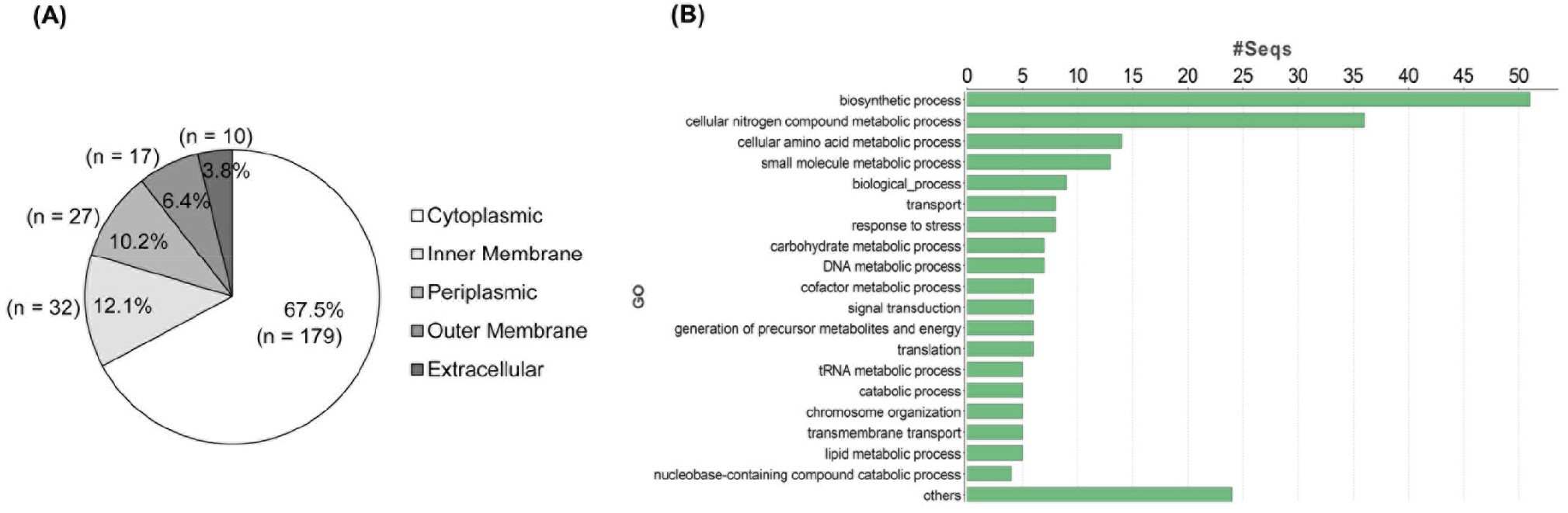 | Fig. 1Proteomic analysis of OMVs isolated from B. cepacia ATCC 25416. Bacterial were cultured in LB broth and OMVs were isolated from culture supernatants. Proteomic analysis was performed using 1-DE-LC-MS/MS. Locations of proteins were predicted using the subcellular location prediction program, CELLO version 2.5 (http://cello.life.nctu.edu.tw/). Proteins identified in B. cepacia OMVs were classified according to Gene Ontology (GO) functions using Blast2Go Pro software (https://www.blast2go.com/). A total of 265 proteins were analyzed by cellular localization (A) and GO (B). |
Table 1.
Major proteins identified in the outer membrane vesicles derived from B. cepacia ATCC 25416 using 1-DE-LC-MS/ MS analysis
Induction of pro-inflammatory response to proteins derived from B. cepacia OMVs
We previously showed that OMVs from B. cepacia ATCC 25416 induced host cell cytotoxicity and pro-inflammatory responses in A549 cells in vitro (20). To determine whether proteins in OMVs were directly responsible for cytotoxicity and pro-inflammatory responses, OMVs were treated with proteinase K and then A549 cells were incubated with either intact OMVs or proteinase K-treated OMVs. Cell death was not different between intact and proteinase K-treated OMVs (Fig. 2A); however, the proteinase K-treated OMVs did not stimulate the expression of pro-inflammatory cytokine genes IL1B and IL6, nor the chemokine genes IL8 and CCL2 (Fig. 2B). The expression of the TNF gene was significantly different between intact and proteinase K-treated OMVs at 10 μg/ml. These results suggest that proteins in B. cepacia OMVs are not responsible for host cell cytotoxicity, but are responsible for pro-inflammatory responses in A549 cells.
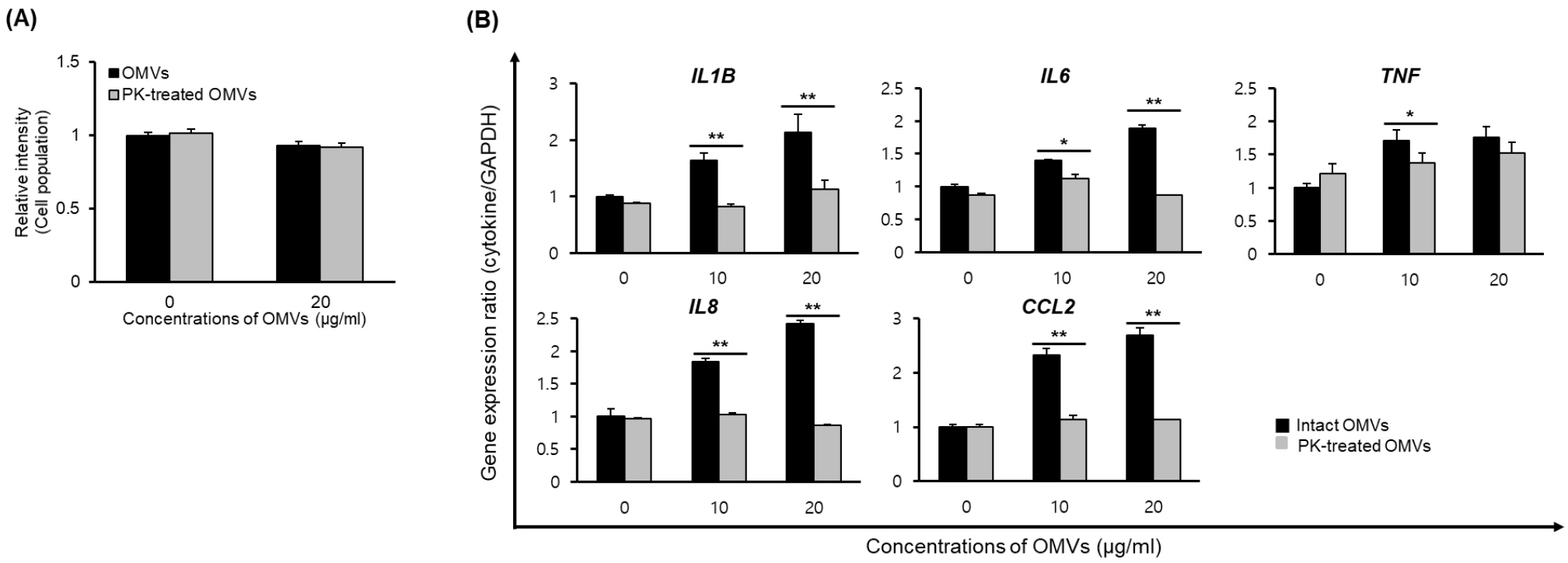 | Fig. 2Host cell responses to proteinase K (PK)-treated B. cepacia OMVs. OMVs were isolated from the culture supernatants of B. cepacia cultured in LB. OMVs were treated with 0.1 μg/mL proteinase K for 3 h at 50°C for the degradation of OMV proteins. A549 cells were treated with either intact OMVs or PK-treated OMVs. (A) Cells were treated with 20 μg/mL OMVs for 24 h and cell viability was determined using MTT assay. Data are presented as mean ± SD of three independent experiments. (B) Cells were treated with various concentrations of OMVs for 6 h, and gene expression was assessed by qPCR. Data are presented as mean ± SD of three independent experiments. *P < 0.05, **P < 0.01 compared to intact OMVs. |
Go to : 
DISCUSSION
B. cepacia ATCC 25416 and two clinical isolates, P1311 and P1383, produced OMVs during in vitro culture (20). These B. cepacia OMVs induced the host cell cytotoxicity and the expression of pro-inflammatory cytokine and chemokine genes in lung epithelial cells, but specific molecules or components associated with the host cell pathology have not been determined. In the present study, we analyzed the proteomes of OMVs isolated from B. cepacia ATCC 25416 and investigated the contribution of OMV proteins to pro-inflammatory responses in vitro. The present study demonstrated that B. cepacia OMVs contained 256 proteins derived from all bacterial compartments, and that proteins in OMVs were directly responsible for pro-inflammatory responses in vitro.
Gram-negative, non-fermenting bacterial pathogens, including B. pseudomallei (28), B. mallei (29), P. aeruginosa (15), A. baumannii (16), A. nosocomialis (17), and S. maltophilia (18), produced OMVs. OMVs derived from gram-negative, non-fermenting pathogens induced host cell cytotoxicity and pro-inflammatory responses. In addition, B. cepacia OMVs induced the cytotoxicity in A549 cells (20), but only < 20% of cells died at 20 μg/ml protein concentrations of B. cepacia OMVs, suggesting that B. cepacia OMVs are cytotoxic to host cells. Azurin homologue and hemolysin were found to be cytotoxic among the secretomes produced by BCC bacteria (30, 31), but proteomic analysis revealed that azurin, azurin homologues, and hemolysin were not identified in B. cepacia OMVs in this study. Moreover, there was no significant difference in host cell cytotoxicity between intact OMVs and proteinase K-treated OMVs, indicating that non-protein components in B. cepacia OMVs may be responsible for the host cell cytotoxicity. Instead, the present study identified several putative virulence-associated proteins in B. cepacia OMVs. Although B. cepacia OMVs are not highly cytotoxic to host cells, it is necessary to clarify cytotoxic factors in B. cepacia OMVs for the development of OMV vaccines against B. cepacia.
We previously demonstrated that OMVs derived from B. cepacia ATCC 25416 induced the expression of pro-inflammatory cytokine genes, including IL1B, IL6, IL8, TNF, and CCL2, in A549 cells, and also induced expression of IL1B, IL6, CXCL1P1, TNF, and CCL2 genes in the lungs of mice injected with B. cepacia OMVs (20). Consistent with the previous study, the present study also showed that OMVs derived from B. cepacia ATCC 25416 induced the expression of pro-inflammatory cytokine and chemokine genes in A549 cells. However, expression of IL1B, IL6, IL8, and CCL2 genes was completely inhibited in A549 cells incubated with proteinase K-treated B. cepacia OMVs (Fig. 2). These results suggest that proteins packaged in B. cepacia OMVs are associated with the expression of these cytokine genes. In the present study, we did not determine the specific proteins associated with pro-inflammatory responses in B. cepacia OMVs. However, our study demonstrates that proteins in B. cepacia OMVs are potent pathogen-associated molecular patterns to stimulate pro-inflammatory responses in vitro. The proteomic analysis of OMVs and the contribution of OMV proteins to host cell pathology may improve our understanding of B. cepacia pathogenesis.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download