INTRODUCTION
The persistent increase in multidrug-resistant (MDR) bacterial infections underscores the demand for new antibiotics. Currently, resistance to antibiotics is a severe global crisis that mostly influences public health (1, 2). The increase in multidrug-resistant (MDR) pathogenic bacteria is increasing the demand for new antibiotics (1, 2). MDR gram-negative bacteria (GNB), such as carbapenem-resistant (CR) Pseudomonas aeruginosa (P. aeruginosa) and Acinetobacter baumannii (A. baumannii), are increasing worldwide. Resistance to third-generation cephalosporins significantly increases the mortality rates of seriously ill patients (3, 4, 5). The World Health Organization (WHO) ranked these resistant GNB as a top priority MDR in 2018 (6).
Due to the unavailability of new antibiotics, the treatment of infections has generally focused on older antimicrobial agents (7, 8). Numerous pharmaceutical industries have dedicated huge research expenditures for the development and discovery of new antibiotics exhibiting superior activity against MDR-GNBs (9). However, there are still a few novel anti-microbial candidates against MDR-GNBs that are in the clinical trial phase (3, 9). Among them, one of the most promising antibiotic candidates is cefiderocol, which has higher potential against MDR-GNBs than older or upcoming antimicrobial agents (9). Cefiderocol is a novel injectable cephalosporin antibiotic that has been newly developed to treat different microorganisms, such as β-lactam-resistant and carbapenem-resistant bacteria (10). Modification of its structure is a useful tool for developing antibiotics via the addition of other moieties that imitate siderophore antibiotics, which are iron-chelating molecules. These molecules are produced by bacteria to capture and distribute iron to bacterial cells for survival (11, 12). Although siderophore antibiotics have been investigated for the last few decades, their clinical development has not progressed due to a lack of correlation between in vitro and in vivo activities or cardiovascular toxicity and low molecular stability (12, 13, 14, 15, 16, 17). A siderophore cephalosporin produces antibacterial activity by inhibiting cell membrane synthesis of gram-negative pathogens by binding to penicillin-binding proteins. Cefiderocol easily penetrates the bacterial periplasmic region due to its siderophore-like activity, a beneficial point for stability against β-lactamases (18). It is also highly stable against extended-spectrum beta-lactamases (ESBL) and class A, B, C, and D carbapenemases (19, 20). This antibiotic has a pyrrolidinium functional group at C-3 similar to cefepime, and a carboxypropanoxyimino functional group at C-7, similar to ceftazidime (18). Thus, cefiderocol possesses strong antibacterial activity against various types of gram-negative bacteria (21).
Carbapenems are effective β-lactam antibacterial agents used to treat high-risk infections associated with different bacteria, including MDR gram-negative bacilli (22). Moreover, cefiderocol is a very effective drug against every type of carbapenemase (23). The combination of potential cell penetration and stability to carbapenemases makes cefiderocol an extremely active drug against microorganisms, especially gram-negative bacteria (23). As an evaluating factor, a phase III clinical study has been completed to manage nosocomial pneumonia and other infections caused by gram-negative microorganisms. This study was conducted by Shionogi Inc. (APEKS-NP), a randomized, double-blind, active-controlled, non-inferiority phase III study (24, 25). Another phase III clinical study among patients with severe infections caused by CR-GNB was evaluated by CREDIBLE-CR (NCT02714595), a randomized, multicenter, open-label phase III clinical investigation of cefiderocol compared with the best available therapy (BAT) (17). Previously, a phase II clinical trial of cefiderocol was performed to determine the clinical safety profile of intravenous injectable cefiderocol compared with imipenem/cilastatin among patients who have complicated urinary tract infections (cUTIs) (18). Aside from a few reported adverse effects related to gastrointestinal side-effects and phlebitis, cefiderocol is well tolerated. The side-effects profile of cefiderocol is better than that of other cephalosporin antibiotics. Cefiderocol helps to treat infections caused by CR and MDR-GNB. It possesses promising antibacterial properties against resistant P. aeruginosa, Stenotrophomonas maltophilia, A. baumannii, and Burkholderia cepacia (18). Recently, the U.S. Food and Drug Administration (FDA) approved cefiderocol as a drug to treat cUTI (26). In addition, cefiderocol is a potential alternative option for treating various infections because of its exclusive mechanism of action, periplasmic penetration capacity, and stability against β-lactamase (18). In the early 1990s, the siderophore cephalosporin (S-9096) was discovered and developed by Shionogi & Co., Ltd (Osaka, Japan) (16, 18). It is a new US FDA approved drug with potential against CRE and drug-resistant non-fermenting GNB (24). Thus, here we review the current information on the structural characteristics, mode of action, pharmacological characteristics, and clinical application of cefiderocol for the future prospective use of cefiderocol.
Go to : 
STRUCTURAL CHARACTERISTICS AND MODE OF ACTION
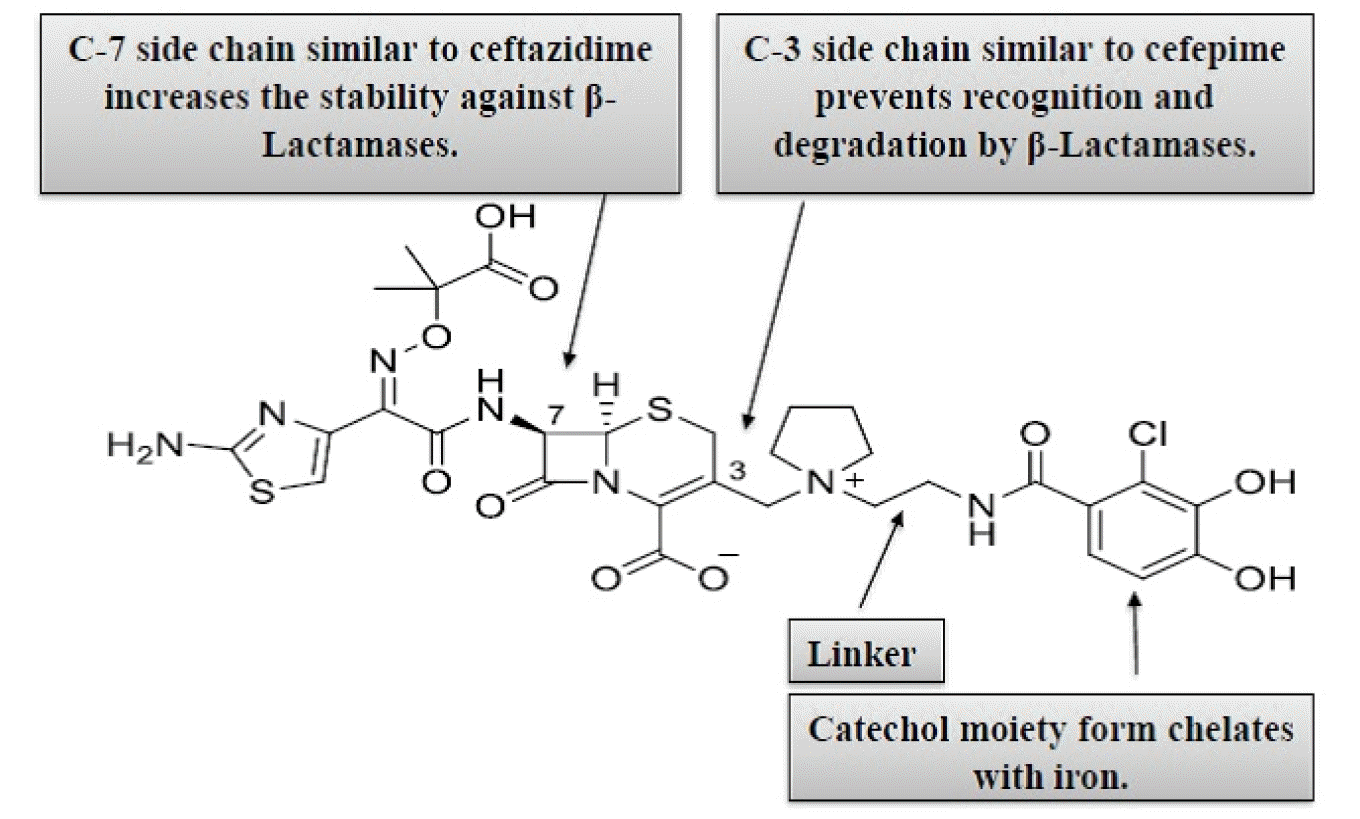
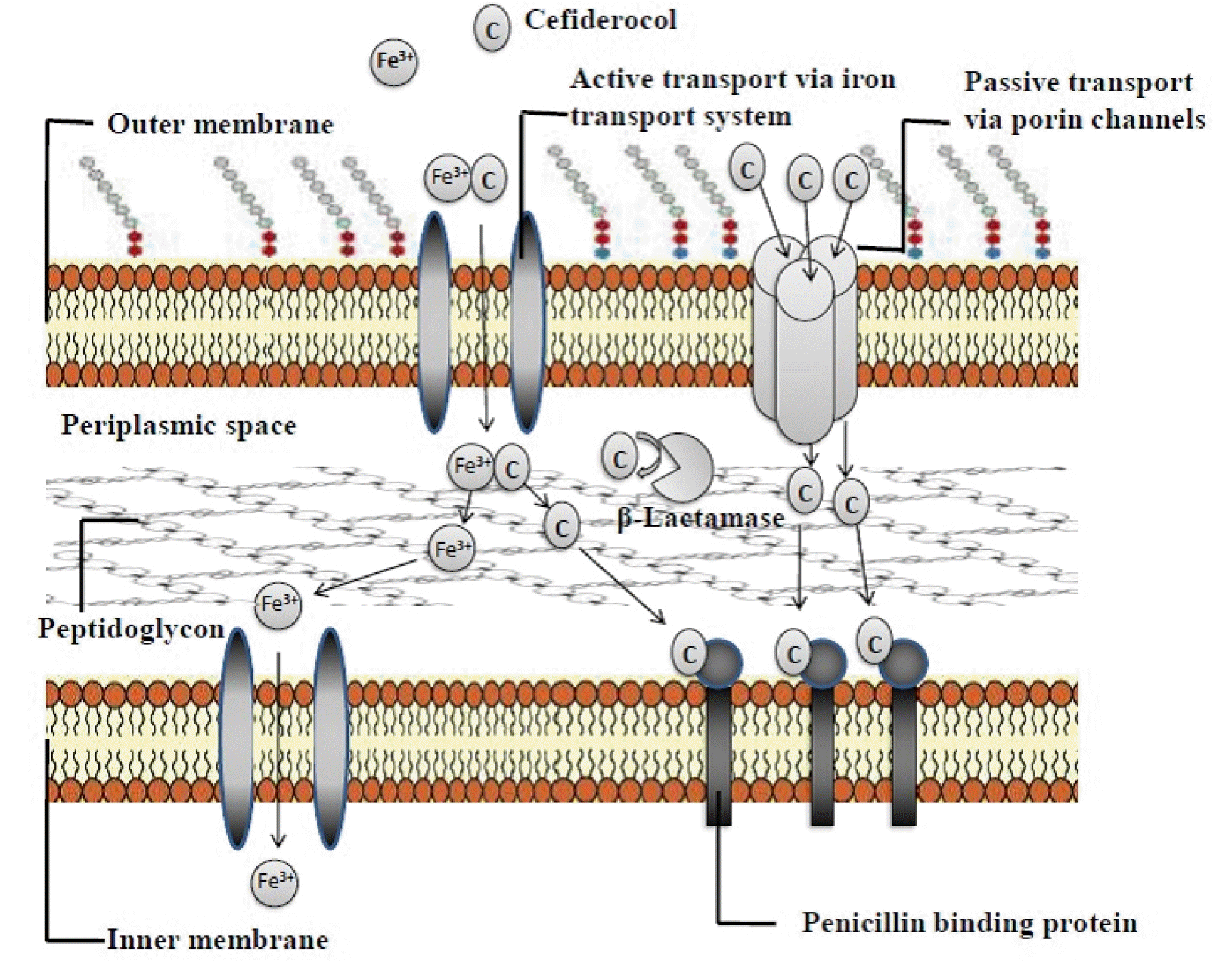
Cefiderocol is a unique catechol-substituted cephalosporin that utilizes bacterial active transport channels to enter the periplasmic space from the outer cell membrane (17). The chemical structure and antibacterial components of cefiderocol are shown in Fig. 1. The potential of cefiderocol is dependent on its extensive stability against different extended-spectrum beta-lactamases (ESBLs), serine-type, and metallo-type carbapenemases (17, 20). Like other β-lactam antibacterial agents, cefiderocol contains a four-membered β-lactam ring bound to a six-member dihydrothiazine ring. Furthermore, the C-3 position of cefiderocol’s side chain includes a catechol moiety, 2-chloro3, 4-dihydroxybenzoic acid, which binds with a linker. The linker is bound by a covalent bond to the pyrrolidine ring (Fig. 1) (18, 27). Cefiderocol has a similar chemical structure to ceftazidime and cefepime. An aminoacyl group with an aminothiadiazole ring and a carboxypropyl-oxyimino chain associated with the α-carbon at the C-7 position of the side chain of cefiderocol is similar to ceftazidime. This aminothiazole ring plays a vital role in enlarging the spectrum of cephalosporins and increasing the effectiveness against pathogens (20, 28).
Moreover, the carboxypropyl-oxyimino group significantly impacts the increased transport through the outer cell membrane and the stability against different β-lactamases. It is liable for the enhanced effectiveness of cefiderocol against gram-negative microorganisms (28, 29). Like cefepime, cefiderocol has a pyrrolidinium functional group at carbon position-3, which increases antibacterial properties and stability against β-lactamases (30). The main difference between cefiderocol and ceftazidime and cefepime is a covalently bound chlorocatechol group with a pyrrolidine ring at position-3, which is responsible for siderophore activity (31). This pyrrolidine ring is responsible for zwitterionic properties that increase water solubility and allow rapid permeability through the outer cell wall of GNBs (28, 32). The C-3 side-chain accounts for the increased accumulation of cefiderocol in the periplasmic space, in contrast to ceftazidime and cefepime (31). The chlorocatechol group of cefiderocol allows chelate formation with ferric iron. Once a chelate complex as a siderophore is formed, the molecule across the cell wall of the gram-negative microorganism and the concentration of cefiderocol increase in the periplasmic region (28, 31). In the periplasmic space, the iron separates from the complex, and then cefiderocol becomes free and binds to penicillin-binding proteins (PBP).
The catechol moiety at the C-3 position of the cefiderocol side chain was enhanced to form chelating complexes with ferric iron. Using the active iron transporter, cefiderocol and siderophore-like molecules have excellent ability to transport extracellular iron into the periplasmic region from the outer cell wall of bacteria, especially gram-negative bacteria (Fig. 2) (31, 33). Chelated iron complexes can penetrate the periplasmic space. Then, the iron atom dissociates from the chelate complex and enters the cytoplasm. In the periplasmic space, cefiderocol is released from chelate complexes and binds with penicillin-binding proteins (PBPs), initially to PBP3, and blocks peptidoglycan synthesis that causes cell death (19, 31, 33). Even though most β-lactams penetrate the outer cell wall of gram-negative microorganisms by passive diffusion to cross porin channels, cefiderocol has an exclusive mechanism of action for its siderophore-like penetration (also known as a Trojan-horse strategy) in periplasmic space and the escape of β-lactamase (18). Another property of its mechanism of action is its stability against almost all β-lactamases that enhance antibacterial properties compared to β-lactamase inhibitors, carbapenems, and other cephalosporins (18, 19). Strong inhibition of PBP3 by cefiderocol was evaluated by comparing half-inhibitory concentrations (IC50s) of cefiderocol (mean IC50 of cefiderocol for PBP3 of Escherichia coli = 0.04 mg/L; mean IC50 for PBP3 of Pseudomonas aeruginosa = 0.06 mg/L) to the IC50s of ceftazidime (mean IC50 for PBP3 of Escherichia coli = 0.45 mg/L; mean IC50 for PBP3 of Pseudomonas aeruginosa = 0.09 mg/L) (33).
Go to : 
PHARMACOLOGICAL CHARACTERISTICS
Cefiderocol has exhibited efficacy against different microorganisms such as P. aeruginosa, Proteus mirabilis, Escherichia coli, and the Enterobacter cloacae complex (34). An in vitro study of multi-national surveillance investigations for numerous gram-negative microorganisms, such as CR strains, has been documented. Among carbapenem-nonsusceptible strains of P. aeruginosa, Enterobacteriaceae, S. maltophilia, and A. baumannii, cefiderocol showed a superior susceptibility ratio compared with ceftolozane-tazobactam, ceftazidime-avibactam, ciprofloxacin, and colistin (16, 33, 35). The U.S. FDA susceptibility breakpoint for cefiderocol is ≤ 1 µg/mL and ≤ 2 µg/mL against P. aeruginosa and Enterobacteriaceae, respectively (34). Cefiderocol has no potential against gram-positive bacteria (34).
Cefiderocol shows time-dependent killing activity similar to other β-lactam antibacterial agents. Pharmacokinetics (PK)/pharmacodynamics (PD) studies predict the effectiveness of cefiderocol based upon the percentage of the frequency of dosing in which unbound drug concentrations are higher than the MIC (%fTMIC) (17, 36, 37). A murine respiratory tract model study illustrates that a longer infusion time of cefiderocol may increase its effectiveness (17, 36). From the above model, the %fTMIC values were 75% and 100% when cefiderocol was administered over 1 h and 3 h, respectively, against a Klebsiella pneumoniae (K. pneumoniae) CR strain, with an MIC of 4 µg/mL (17, 36). In a murine thigh and lung infection model involving P. aeruginosa strains, %fTMIC needed for a static effect, and the 1 − log10 diminution were similar for cefiderocol and cefepime. Static effects and 1 − log10 diminution were observed at 47% and 57%, respectively, for cefiderocol, and 61% and 87%, respectively, for cefepime (17, 37).
Cefiderocol exhibits strong efficacy against different gram-negative pathogens in vitro and preclinical studies of microbial infection. Saisho et al. stated that single and multiple intravenous administration of cefiderocol is well tolerated up to 2,000 mg in healthy subjects (38). There were no severe adverse effects observed in either single or multiple doses of the study. The half-life was observed from 1.98 to 2.74 h. It is mainly excreted unchanged in the urine (approximately 61.5% to 68.4% of the dose (38). The safety profile of cefiderocol was assessed for renal function impairment in another study (39). In this study, the assessment of a 1,000‐mg single intravenous infusion of cefiderocol was performed. The total drug clearance (CL) and half-life (t1/2) of cefiderocol correlated with renal function. Around 60% of cefiderocol was eliminated by hemodialysis for 3 to 4 hours. The plasma‐protein‐unbound portion was comparable between different renal function groups (39). The occurrence of adverse effects did not appear to be correlated with the extent of renal function impairment. A single intravenous dose of 1,000 mg cefiderocol was usually well-tolerated in subjects with impaired renal function. Taken together, renal function impairment affected clearance (CL) and half-life (t1/2) without affecting maximum plasma concentration (Cmax) (39).
Pharmacokinetic-pharmacodynamic (PK/PD) investigations have shown that cefiderocol must be administered thrice daily (every 8 h). The main demographic reasons for the clinical significance in connection with dosage adjustment are renal function and body weight (40). The recommended dose of cefiderocol is 2 g intravenously thrice daily for patients with a creatinine clearance (CLcr) within 60 to 119 mL/min (17, 40). The proposed treatment time with cefiderocol was 7-14 days. The period of treatment depends on the intensity of the infections and the clinical status of patients up to 14 days. Dosage adjustment is necessary for patients with renal impairment (patients with CLcr < 60 mL/min or > 120 mL/min) and end-stage renal disease (ESRD) (17). The suggested dosage for patients with a CLcr of 30 and 59 mL/min is 1.5 mg thrice daily (every 8 h) and 1 g thrice daily (every 8 h) for patients with a CLcr limit of 15–29 mL/min. Administration of 750 mg twice daily (every12 hrs) for patients with ESRD in the presence or absence of intermittent hemodialysis has been proposed (17, 40). In every case, the suggested infusion time is at least 3 hours (41). For patients with CLcr, ≥120 mL/min administration of 2 g twice daily (every 12 h) by intravenous infusion over 3 h is recommended.
Cefiderocol is supplied as a sterile lyophilized powder. Before IV infusion, it should be reconstituted as well as subsequently diluted using an aseptic technique. Cefiderocol is supplied in clear white to off-white single-use glass vials containing 1 g of cefiderocol per vial (17). The cefiderocol is compatible with either 0.9% sodium chloride (NaCl) injection, USP, or 5% dextrose injection, USP. Cefiderocol powder must be reconstituted with either 10 mL of 0.9% sodium chloride (NaCl) for injection or 5% dextrose injection, with the suitable volume subsequently diluted in 100 mL of either sodium chloride or 5% dextrose. The storage duration of reconstituted cefiderocol and diluted cefiderocol are 1 h and 4 h, respectively, at room temperature (17).
Go to : 
RESISTANCE, ADVERSE EFFECTS AND DRUG INTERACTIONS
Limited information is available regarding the resistance mechanism in clinical isolates. In gram-negative bacteria, β-Lactam and carbapenem resistance increase via different mechanisms, such as PBP mutations, β-lactamases, efflux, and permeability reduction due to the loss of porin or mutation (18). Ito et al. evaluated P. aeruginosa isolates’ resistance and evaluated the resistance mechanism of cefiderocol (17). This study illustrated that the resistance of different mutants to cefiderocol was less than that of ceftazidime at different doses, which were four- and ten-fold higher than the MIC of the mentioned agents. The resistance mechanism was observed due to mutations in the iron transporter genes, but cross-resistance was not exhibited with ceftazidime (17). Kohira et al. investigated a ten-generation serial passage investigation utilizing cefiderocol, ceftazidime, and meropenem with three strains of K. pneumoniae and two P. aeruginosa strains (18). The MICs of cefiderocol are increased by 4-fold or less against all of these strains (18). Another recent investigation evaluated the resistance mechanism of isolates showing in the SIDERO-CR-2014/2016 that the MIC of cefiderocol was greater than or equal to 8 µg/mL. The addition of avibactam, an inhibitor of serine β-lactamase, with or without dipicolinic acid, an inhibitor of metallo-β-lactamase inhibitor, exhibited a lower MIC against the mentioned resistant isolates (17).
Cefiderocol is contraindicated for patients with severe hypersensitivity to cefiderocol or other β-lactam antibiotics due to the recognized cross-reactivity of β-lactam antibiotics (17). The cross-reactivity study of cefiderocol and ceftazidime/ cefepime is not available. Therefore, precautions should be taken in patients allergic to ceftazidime/cefepime upon subsequent replacement by cefiderocol antibiotic therapy (17). There is a warning that cefiderocol is associated with an enhanced mortality rate in patients with CRE infections. Further warnings regarding Clostridioides difficile-associated diarrhea (CDAD), as well as the development of drug-resistant microorganisms, have been issued (17). The most frequent adverse effects of cefiderocol are hypertension (4%), diarrhea (4%), headache (3%), infusion site pain (3%), constipation (3%), cough (2%), nausea (2%), and vomiting (2%) (42).
A clinical investigation was recently performed to evaluate the potential inhibition of cefiderocol on drug transporters (17, 43). When cefiderocol was co-administered with furosemide and metformin, the Cmax and area under the plasma concentration-time curve (AUC) were unchanged, but when co-administered with rosuvastatin, the Cmax and AUC0-inf of rosuvastatin were slightly increased but was not considered significant (17, 43). Cefiderocol has no significant drug-drug interaction effects via drug transporters (43).
Go to : 
CLINICAL APPLICATION
Cefiderocol, a potent injectable cephalosporin, is effective against various gram-negative microorganisms. Its exclusive action allows high penetration in the periplasmic space and increases the stability of various β-lactamases (24). Moreover, a dose of 2 g as a 3-h infusion thrice daily has been selected based on pharmacodynamic analysis (24). In vivo studies regarding the efficiency of cefiderocol were investigated in different animal infection models and were deemed superior to or similar compared with other drugs, including ciprofloxacin, tigecycline, and cefepime. Therefore, cefiderocol holds a critical place in therapy of cUTI, especially against infectious gram-negative bacterial disease (24). Few adverse effects and drug-drug interactions and the ability to widely escape from all three CR mechanisms by gram-negative microorganisms make cefiderocol a promising antibiotic in the antibiotic armamentarium (24). It remains unclear whether cefiderocol is appropriate for treating serious systemic infections, including pneumonia, and bloodstream infections (BSI) (24).
On 14th November 2019, the FDA approved cefiderocol, as a siderophore-cephalosporin conjugate antibiotic for the treatment of adult patients with cUTIs, including kidney infections initiated by vulnerable gram-negative microorganisms, when patients have no or rare options for treatment (44). The FDA approval for treatment was based on various preclinical and clinical experimental data, such as in vitro and in vivo studies, along with a pharmacokinetic and pharmacodynamic investigation that demonstrated the potential of cefiderocol as a treatment for cUTI (44).
Go to : 
CONCLUSION
The persistence of multi-resistant and excessively resistant gram-negative microorganisms is a global health problem and underscores the essential need for new antibiotics (45, 46). Cefiderocol has been developed for targeting P. aeruginosa, Enterobacteriaceae, A. baumannii, and S. maltophilia (19, 47). Cefiderocol has recently received approval from the US FDA for the treatment of patients with cUTIs. Thus, physicians now have an alternative antibiotic option for treating adult patients with different infections caused by various resistant microorganisms. Cefiderocol was well-tolerated in a clinical study of patients with cUTIs (17). Thus, cefiderocol holds a crucial place in cUTI therapy, but further studies are required to establish its application in other serious systemic infections, including pneumonia and bloodstream infections (24). Finally, cefiderocol is a potential new antibiotic owing to its novel mechanism of action, higher intracellular penetration capacity into the periplasmic space, and better stability against β-lactamases.
Go to : 




 PDF
PDF Citation
Citation Print
Print


 XML Download
XML Download