INTRODUCTION
METHODS
Materials
Animals
Preparation and extraction of intestinal contents for in vitro study
Determination of intestinal microbial enzyme activity
Analytical conditions
Analytical validation
Stability studies
Intestinal microbiota-mediated metabolism of voglibose in vitro
Pharmacodynamics of voglibose in non-diabetic mice
Pharmacodynamics of voglibose in diabetic mice
RESULTS
Development and optimization of analytical method
Fig. 1
Mass spectra of (A) voglibose with its structure and (B) telmisartan, an internal standard. cps, counts per second; m/z, mass to charge.
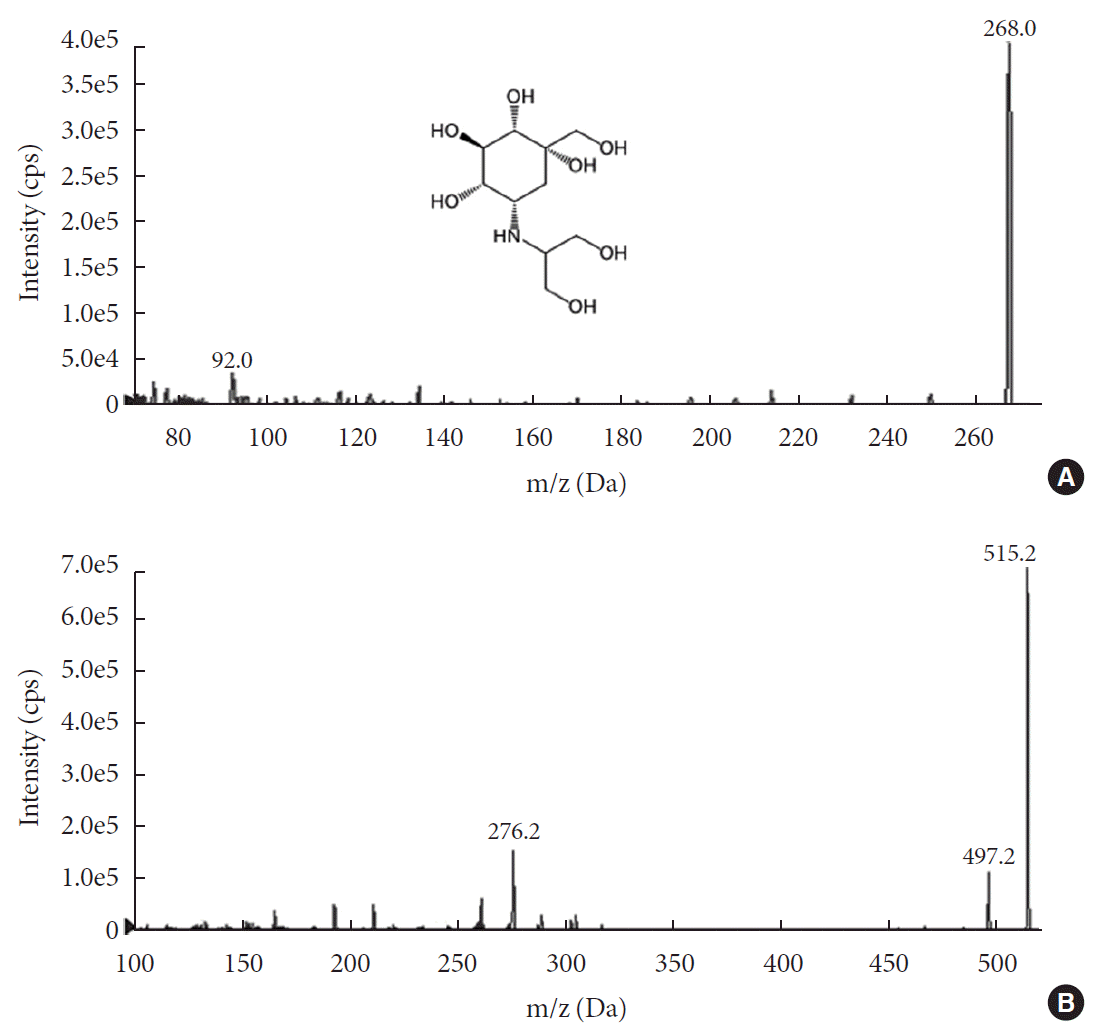
Fig. 2
Representative liquid chromatography-mass spectrometry (LC-MS/MS) chromatograms of (A, B, C) voglibose and (D, E, F) telmisartan. (A, D) blank samples; (B, E) blank samples spiked with analyte at lower limit of quatitation; and (C, F) real samples from in vitro incubation with intestinal contents for 3 hours. cps, counts per second.
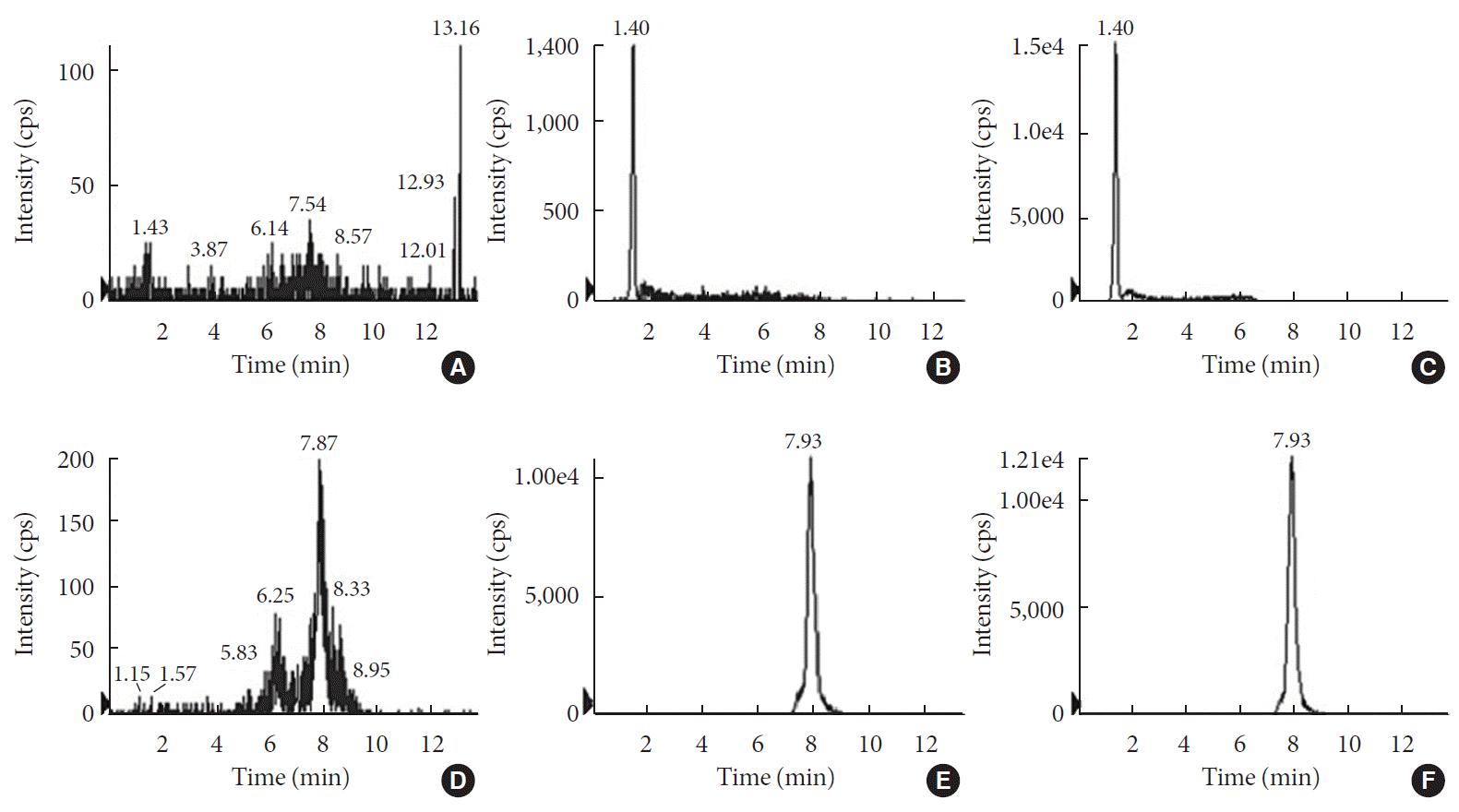
Table 1.
Values are presented as mean±standard deviation (n=5). Short-term stability tests were conducted at 25°C and 37°C for 24 hours. Long-term stability tests were conducted after storing voglibose in freezer and refrigerator (4°C) for 30 and 5 days, respectively. Three freeze-thaw cycles were tested for freeze-thaw stability tests from −20°C to room temperature.
In vitro metabolism of voglibose
Fig. 3
Enzyme activities of intestinal contents: 0.4 mL of either 2.5 mM 4-nitrophenyl β-D-glucopyranoside, 4-nitrophenyl β-D-glucuronide, or 4-nitrophenyl-sulfate in potassium phosphate buffer, pH 7.4, was mixed with 0.4 mL of potassium phosphate buffer, and 0.2 mL of intestinal contents prepared from both antibiotics- and vehicle-treated mice and incubated at 37℃ for 30 minutes. The enzyme activities were calculated following measuring the sample's absorbance at 405 nm. Each bar represents the mean activity of respective enzymes+standard deviation of triplicate determinations. aThe value significantly different from corresponding vehicle-treated controls at P<0.05.
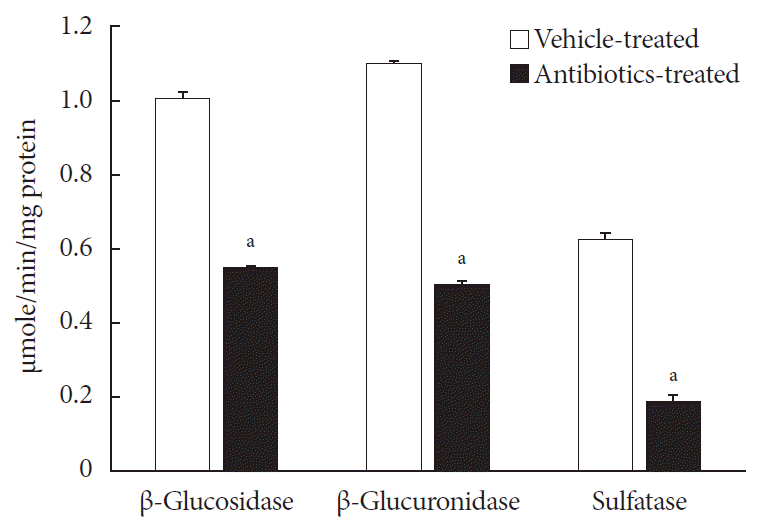
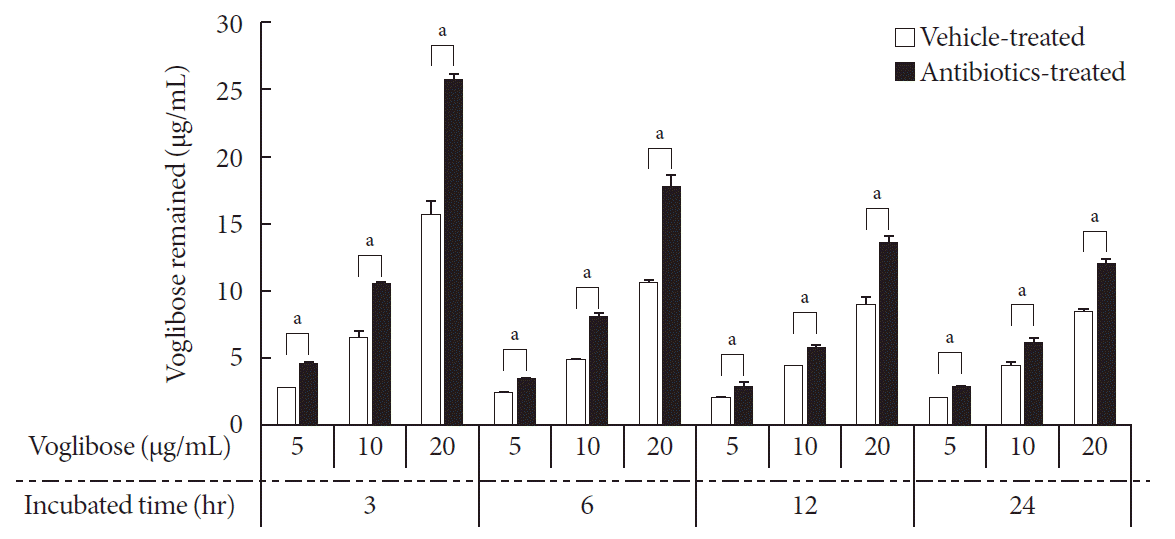
Fig. 4
Time- and concentration-dependent metabolism of voglibose by intestinal microbiota in vitro. Various concentrations of voglibose was incubated with 0.5 g/mL intestinal contents prepared from both antibiotics- and vehicle-treated mice and incubated at 37℃ for various time points. The remained voglibose over time was measured by liquid chromatography-mass spectrometry (LC-MS/MS). Each bar represents the mean concentration of voglibose in the incubated sample+standard deviation of triplicate determinations. aThe value significantly different from corresponding vehicle-treated controls at P<0.05.
Pharmacodynamics of voglibose in normal and diabetic mice
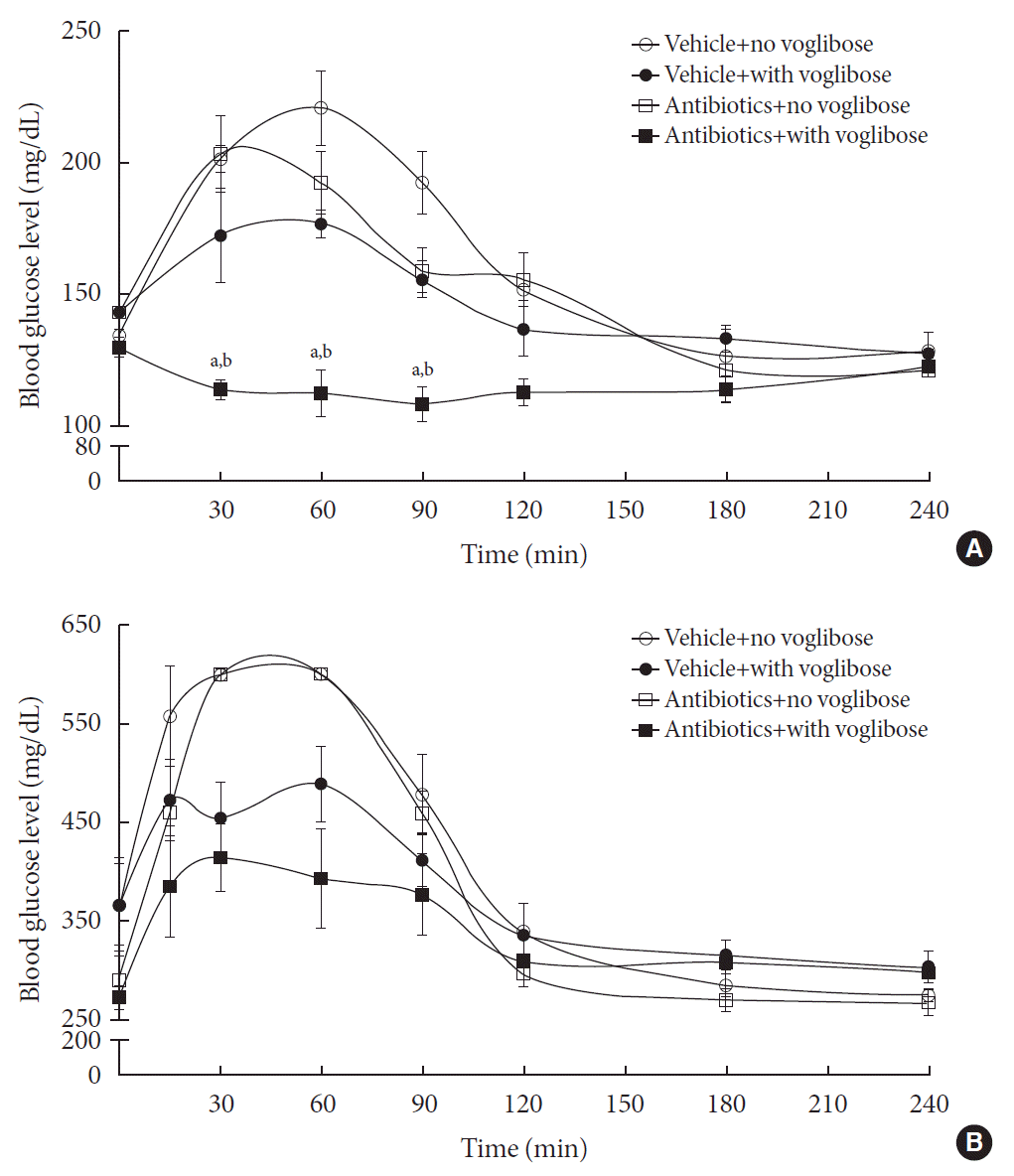
Fig. 5
Blood glucose level in vehicle-treated and antibiotics-treated non-diabetic normal mice (A) and diabetic mice (B). Six-hr fasted vehicle- and antibiotics-treated non-diabetic and diabetic mice were administered with voglibose followed by an oral administration with starch. And then, the change in the blood glucose level was measured at various time points. Each point represents mean blood glucose level±standard deviation of five animals. aThe value indicates significant difference between antibiotics-treated starch only and antibiotics-treated starch+voglibose groups, bThe value indicates significant difference between vehicle-treated starch+voglibose and antibiotics-treated starch+voglibose groups.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download