1. Schonfield WA, Beebe GW. Normal growth and variation in the male genitalia from birth to maturity. J Urol. 1942; 48:759–77.
2. Hatipoğlu N, Kurtoğlu S. Micropenis: etiology, diagnosis and treatment approaches. J Clin Res Pediatr Endocrinol. 2013; 5:217–23.
3. Feldman KW, Smith DW. Fetal phallic growth and penile standards for newborn male infants. J Pediatr. 1975; 86:395–8.

4. Makiyan Z. Systematization of ambiguous genitalia. Organogenesis. 2016; 12:169–82.

5. Marshall FF. Embryology of the lower genitourinary tract. Urol Clin North Am. 1978; 5:3–15.

6. Shen J, Cunha GR, Sinclair A, Cao M, Isaacson D, Baskin L. Macroscopic whole-mounts of the developing human fetal urogenital-genital tract: indifferent stage to male and female differentiation. Differentiation. 2018; 103:5–13.

7. Migeon CJ, Wisniewski AB. Congenital adrenal hyperplasia owing to 21-hydroxylase deficiency. Growth, development, and therapeutic considerations. Endocrinol Metab Clin North Am. 2001; 30:193–206.
8. Cheng PK, Chanoine JP. Should the definition of micropenis vary according to ethnicity? Horm Res. 2001; 55:278–81.

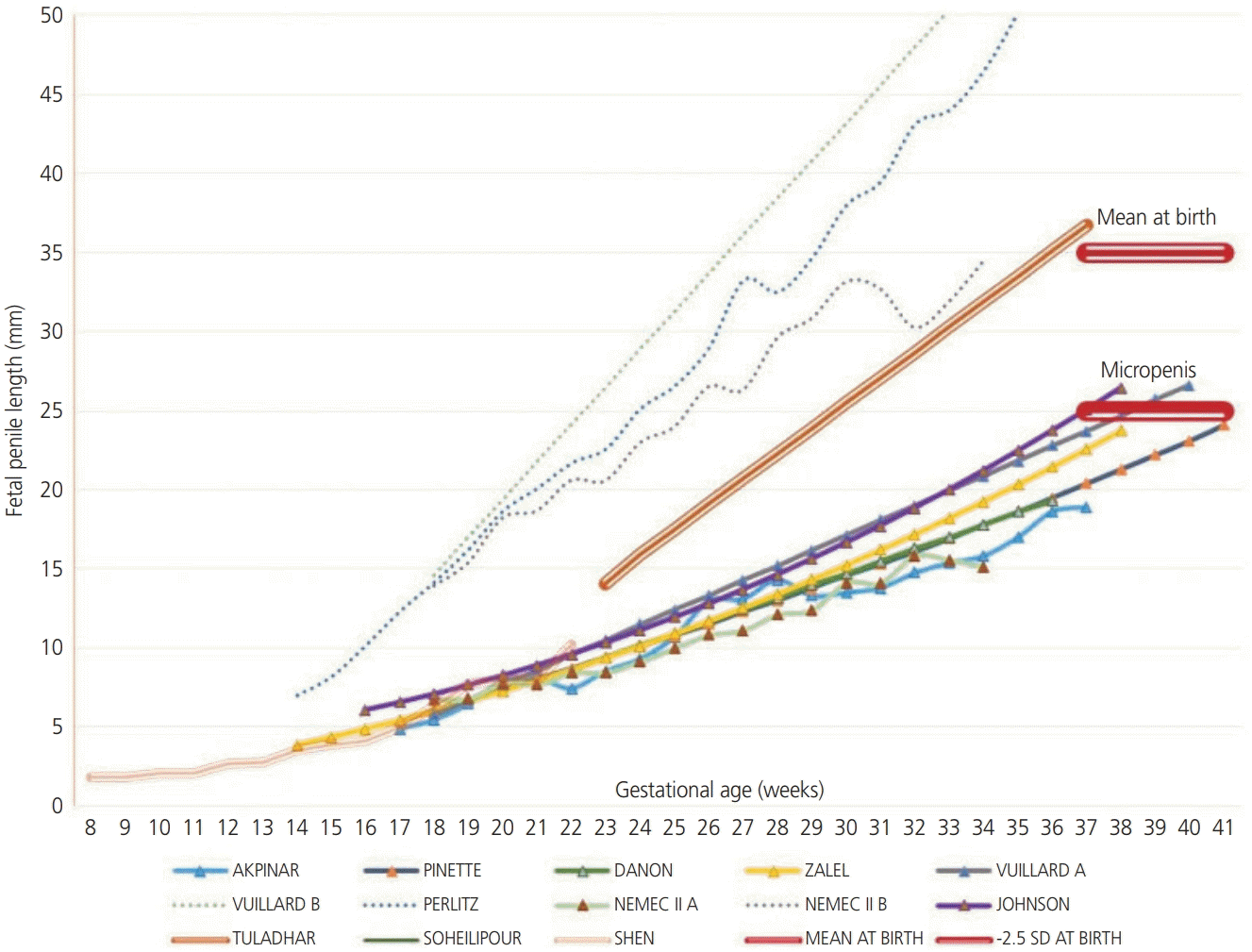
9. Zalel Y, Pinhas-Hamiel O, Lipitz S, Mashiach S, Achiron R. The development of the fetal penis--an in utero sonographic evaluation. Ultrasound Obstet Gynecol. 2001; 17:129–31.
10. Lin SK, Lee YH, Pong HC, Ho ES. Prenatal diagnosis of a rare variant of hypospadias and review of the literature. Ultrasound Obstet Gynecol. 2001; 18:678–80.

11. Yinon Y, Kingdom JCP, Proctor LK, Kelly EN, Salle JLP, Wherrett D, et al. Hypospadias in males with intrauterine growth restriction due to placental insufficiency: the placental role in the embryogenesis of male external genitalia. Am J Med Genet A. 2010; 152A:75–83.

12. Nemec SF, Nemec U, Weber M, Brugger PC, Bettelheim D, Rotmensch S, et al. Penile biometry on prenatal magnetic resonance imaging. Ultrasound Obstet Gynecol. 2012; 39:330–5.

13. Winter RM, Baraitser M. The London dysmorphology database. 5th ed. Oxford: Oxford University Press;2001.
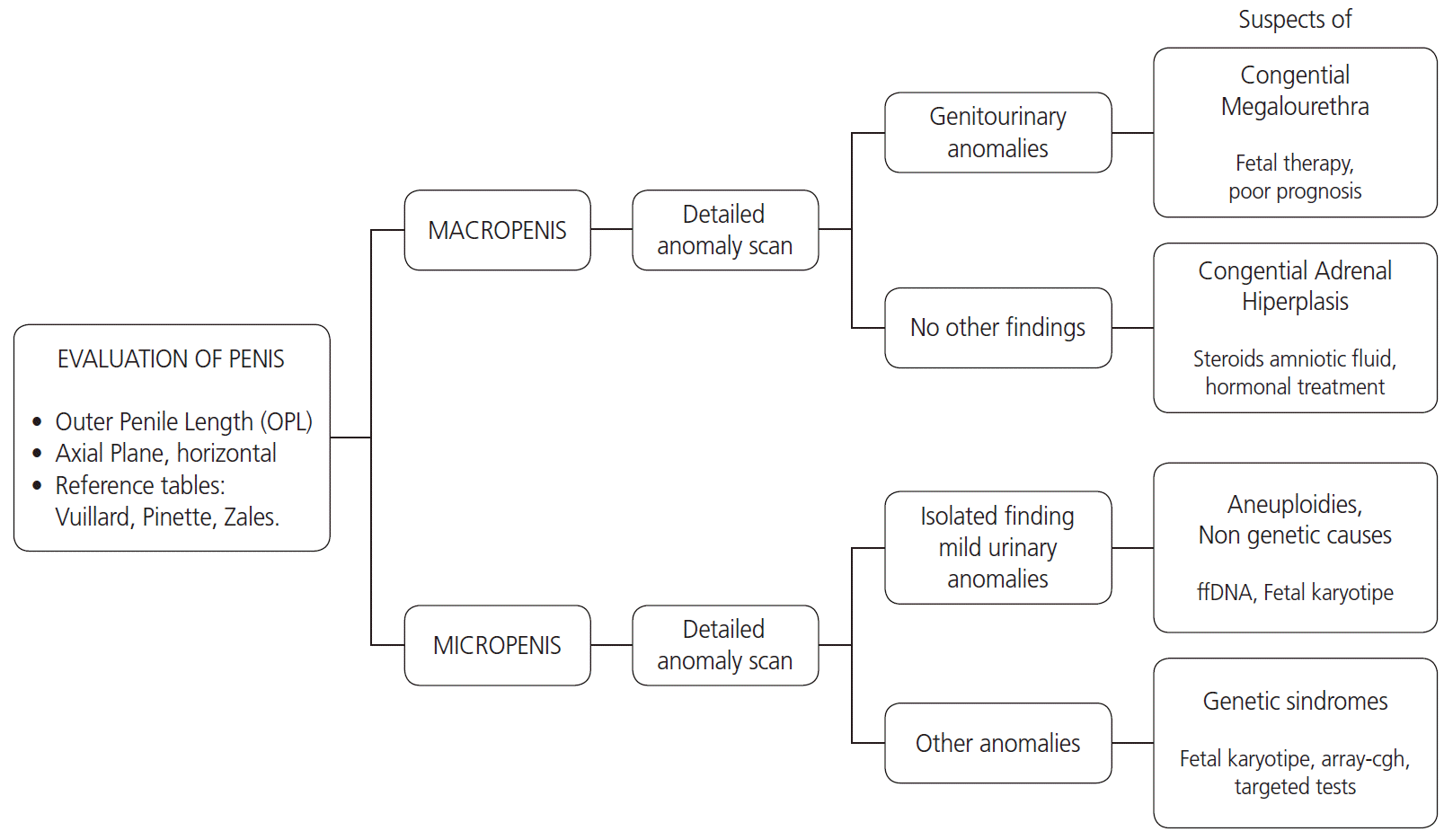
14. Moaddab A, Sananes N, Hernandez-Ruano S, Werneck Britto IS, Blumenfeld Y, Stoll F, et al. Prenatal diagnosis and perinatal outcomes of congenital megalourethra: a multicenter cohort study and systematic review of the literature. J Ultrasound Med. 2015; 34:2057–64.
15. Wax JR, Pinette MG, Landes A, Cartin A, Blackstone J. Prenatal sonographic diagnosis of congenital megalourethra with in utero spontaneous resolution. J Ultrasound Med. 2009; 28:1385–8.

16. Huynh T, McGown I, Cowley D, Nyunt O, Leong GM, Harris M, et al. The clinical and biochemical spectrum of congenital adrenal hyperplasia secondary to 21-hydroxylase deficiency. Clin Biochem Rev. 2009; 30:75–86.
17. Pajkrt E, Petersen OB, Chitty LS. Fetal genital anomalies: an aid to diagnosis. Prenat Diagn. 2008; 28:389–98.

18. Merke DP, Bornstein SR. Congenital adrenal hyperplasia. Lancet. 2005; 365:2125–36.

19. Wessells H, Lue TF, McAninch JW. Penile length in the flaccid and erect states: guidelines for penile augmentation. J Urol. 1996; 156:995–7.

20. Kollurage UA, Atapattu N, Jayamanne BD, Gunasiri JR, de Silva SH. Assessment of the stretched penile length in Sri Lankan newborns. Ceylon Med J. 2019; 64:4–8.

21. Danon D, Ben-Shitrit G, Bardin R, Machiach R, Vardimon D, Meizner I. Reference values for fetal penile length and width from 22 to 36 gestational weeks. Prenat Diagn. 2012; 32:829–32.

22. Rotondi M, Valenzano F, Bilancioni E, Spanò G, Rotondi M, Giorlandino C. Prenatal measurement of testicular diameter by ultrasonography: development of fetal male gender and evaluation of testicular descent. Prenat Diagn. 2001; 21:112–5.

23. Vuillard E, Chitrit Y, Dreux S, Elghoneimi A, Oury JF, Muller F. Sonographic measurement of corpus spongiosum in male fetuses. Prenat Diagn. 2011; 31:1160–3.

24. Akpinar F, Yilmaz S, Akdag Cirik D, Kayikcioglu F, Dilbaz B, Yucel H, et al. Sonographic assessment of the fetal penile development. Fetal Pediatr Pathol. 2016; 35:88–92.

25. Pinette MG, Wax JR, Blackstone J, Cartin A. Normal growth and development of fetal external genitalia demonstrated by sonography. J Clin Ultrasound. 2003; 31:465–72.

26. Johnson P, Maxwell D. Fetal penile length. Ultrasound Obstet Gynecol. 2000; 15:308–10.

27. Tuladhar R, Davis PG, Batch J, Doyle LW. Establishment of a normal range of penile length in preterm infants. J Paediatr Child Health. 1998; 34:471–3.

28. Radhakrishnan J, Razzaq A, Manickam K. Concealed penis. Pediatr Surg Int. 2002; 18:668–72.

29. Perlitz Y, Keselman L, Haddad S, Mukary M, Izhaki I, Ben-Ami M. Prenatal sonographic evaluation of the penile length. Prenat Diagn. 2011; 31:1283–5.

30. Jakobovits AA. Fetal penile erection. Ultrasound Obstet Gynecol. 2001; 18:405.

31. Vasudevan G, Manivarmane R, Bhat BV, Bhatia BD, Kumar S. Genital standards for south Indian male newborns. Indian J Pediatr. 1995; 62:593–6.

32. Ponchietti R, Mondaini N, Bonafè M, Di Loro F, Biscioni S, Masieri L. Penile length and circumference: a study on 3,300 young Italian males. Eur Urol. 2001; 39:183–6.
33. Fok TF, Hon KL, So HK, Wong E, Ng PC, Chang A, et al. Normative data of penile length for term Chinese newborns. Biol Neonate. 2005; 87:242–5.

34. Al-Herbish AS. Standard penile size for normal full term newborns in the Saudi population. Saudi Med J. 2002; 23:314–6.
35. Lian WB, Lee WR, Ho LY. Penile length of newborns in Singapore. J Pediatr Endocrinol Metab. 2000; 13:55–62.

36. Soheilipour F, Rohani F, Dehkordi EH, Isa Tafreshi R, Mohagheghi P, Zaheriani SM, et al. The nomogram of penile length and circumference in Iranian term and preterm neonates. Front Endocrinol (Lausanne). 2018; 9:126.

37. Bhakhri BK, Meena SS, Rawat M, Datta V. Neonatal stretched penile length: relationship with gestational maturity and anthropometric parameters at birth. Paediatr Int Child Health. 2015; 35:53–5.

38. Sharony R, Bental YA, Eyal O, Biron-Shental T, Weisbrod M, Shiff Y, et al. Correlation between prenatal and postnatal penile and clitoral measurements. J Clin Ultrasound. 2012; 40:394–8.

39. Katorza E, Pinhas-Hamiel O, Mazkereth R, Gilboa Y, Achiron R. Sex differentiation disorders (SDD) prenatal sonographic diagnosis, genetic and hormonal work-up. Pediatr Endocrinol Rev. 2009; 7:12–21.
40. Salomon LJ, Alfirevic Z, Berghella V, Bilardo C, Hernandez-Andrade E, Johnsen SL, et al. Practice guidelines for performance of the routine mid-trimester fetal ultrasound scan. Ultrasound Obstet Gynecol. 2011; 37:116–26.

41. Reddy UM, Abuhamad AZ, Levine D, Saade GR; Fetal Imaging Workshop Invited Participants. Fetal imaging: executive summary of a joint Eunice Kennedy Shriver National Institute of Child Health and Human Development, Society for Maternal-Fetal Medicine, American Institute of Ultrasound in Medicine, American College of Obstetricians and Gynecologists, American College of Radiology, Society for Pediatric Radiology, and Society of Radiologists in ultrasound fetal imaging workshop. Obstet Gynecol. 2014; 123:1070–82.
42. Anh DD, Nguyen HT, Meagher S, Araujo Júnior E. Prenatal diagnosis of congenital megalourethra in the second trimester of pregnancy. J Ultrason. 2019; 19:302–4.

43. Khanal D, Mandal D, Phuyal R, Adhikari U. Congenital adrenal hyperplasia with salt wasting crisis: a case report. JNMA J Nepal Med Assoc. 2020; 58:56–8.

44. Lee PA, Houk CP, Ahmed SF, Hughes IA; International Consensus Conference on Intersex organized by the Lawson Wilkins Pediatric Endocrine Society and the European Society for Paediatric Endocrinology. Consensus statement on management of intersex disorders. International Consensus Conference on Intersex. Pediatrics. 2006; 118:e488–500.
45. Epelboym Y, Estrada C, Estroff J. Ultrasound diagnosis of fetal hypospadias: accuracy and outcomes. J Pediatr Urol. 2017; 13:484.e1–4.

46. Ahmed SF, Achermann JC, Arlt W, Balen A, Conway G, Edwards Z, et al. Society for Endocrinology UK guidance on the initial evaluation of an infant or an adolescent with a suspected disorder of sex development (revised 2015). Clin Endocrinol (Oxf). 2016; 84:771–88.
47. Nef S, Neuhaus TJ, Spartà G, Weitz M, Buder K, Wisser J, et al. Outcome after prenatal diagnosis of congenital anomalies of the kidney and urinary tract. Eur J Pediatr. 2016; 175:667–76.

48. Graziani RNA, Nemzer L, Kerns J. The experience of genetic counselors working with patients facing the decision of pregnancy termination after 24 weeks gestation. J Genet Couns. 2018; 27:626–34.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download