1. Alkema L, Chou D, Hogan D, Zhang S, Moller AB, Gemmill A, et al. Global, regional, and national levels and trends in maternal mortality between 1990 and 2015, with scenario-based projections to 2030: a systematic analysis by the UN Maternal Mortality Estimation InterAgency Group. Lancet. 2016; 387:462–74.

2. von Herrath MG, Oldstone MB. Virus-induced autoimmune disease. Curr Opin Immunol. 1996; 8:878–85.
3. Say L, Chou D, Gemmill A, Tunçalp Ö, Moller AB, Daniels J, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health. 2014; 2:e323–33.

4. Romero-Gutiérrez G, Espitia-Vera A, Ponce-Ponce de León AL, Huerta-Vargas LF. Risk factors of maternal death in Mexico. Birth. 2007; 34:21–5.

5. Maraschini A, Lega I, D’Aloja P, Buoncristiano M, Dell’Oro S, Donati S, et al. Women undergoing peripartum hysterectomy due to obstetric hemorrhage: a prospective population-based study. Acta Obstet Gynecol Scand. 2020; 99:274–82.

6. Rouse DJ, MacPherson C, Landon M, Varner MW, Leveno KJ, Moawad AH, et al. Blood transfusion and cesarean delivery. Obstet Gynecol. 2006; 108:891–7.

7. World Health Organization. Guideline: delayed umbilical cord clamping for improved maternal and infant health and nutrition outcomes. Geneva: World Health Organization;2014.
8. Begley CM, Gyte GM, Devane D, McGuire W, Weeks A, Biesty LM. Active versus expectant management for women in the third stage of labour. Cochrane Database Syst Rev. 2019; 2:CD007412.

9. Frolova AI, Stout MJ, Tuuli MG, López JD, Macones GA, Cahill AG. Duration of the third stage of labor and risk of postpartum hemorrhage. Obstet Gynecol. 2016; 127:951–6.

10. Say L, Souza JP, Pattinson RC; WHO working group on Maternal Mortality and Morbidity classifications. Maternal near miss--towards a standard tool for monitoring quality of maternal health care. Best Pract Res Clin Obstet Gynaecol. 2009; 23:287–96.
11. Byun JM, Kim YN, Jeong DH, Seo YJ, Jeong EJ, Kang JY, et al. Prognosis and indication of emergency hysterectomy following postpartum hemorrhage. Korean J Obstet Gynecol. 2012; 55:901–6.

12. Mercier FJ, Van de Velde M. Major obstetric hemorrhage. Anesthesiol Clin. 2008; 26:53–66.

13. Drucker M, Wallach RC. Uterine packing: a reappraisal. Mt Sinai J Med. 1979; 46:191–4.
14. Bakri YN, Amri A, Abdul Jabbar F. Tamponade-balloon for obstetrical bleeding. Int J Gynaecol Obstet. 2001; 74:139–42.

15. Tamizian O, Arulkumaran S. The surgical management of postpartum haemorrhage. Curr Opin Obstet Gynecol. 2001; 13:127–31.

16. B-Lynch C, Coker A, Lawal AH, Abu J, Cowen MJ. The B‐Lynch surgical technique for the control of massive postpartum haemorrhage: an alternative to hysterectomy? Five cases reported. BJOG. 1997; 104:372–5.

17. Yucel O, Ozdemir I, Yucel N, Somunkiran A. Emergency peripartum hysterectomy: a 9-year review. Arch Gynecol Obstet. 2006; 274:84–7.

18. Hutton B, Salanti G, Caldwell DM, Chaimani A, Schmid CH, Cameron C, et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: checklist and explanations. Ann Intern Med. 2015; 162:777–84.

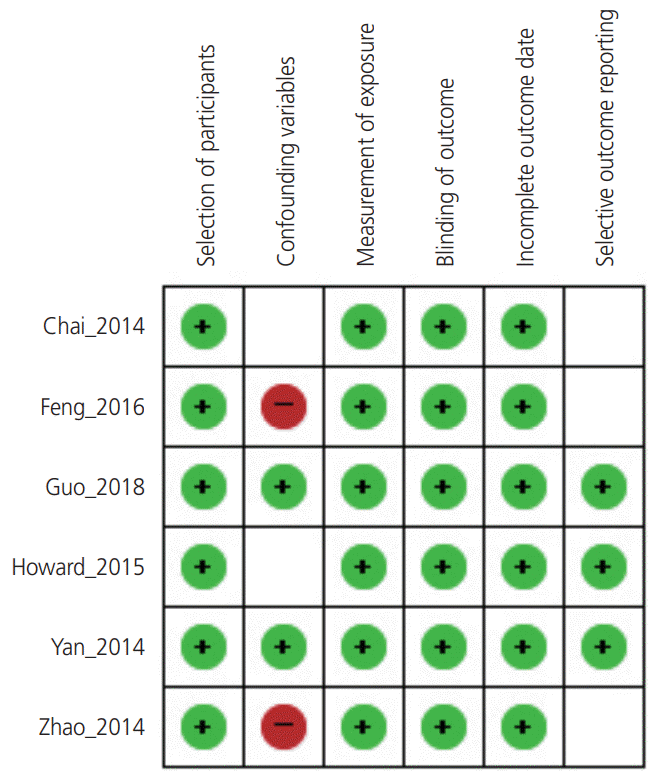
19. Chai VY, To WW. Uterine compression sutures for management of severe postpartum haemorrhage: five-year audit. Hong Kong Med J. 2014; 20:113–20.
20. Feng B, Zhai J, Cai Y. Results of surgical intervention in treating post-cesarean intractable postpartum hemorrhage. Int J Clin Exp Med. 2016; 9:5154–60.
21. Guo Y, Hua R, Bian S, Xie X, Ma J, Cai Y, et al. Intrauterine Bakri balloon and vaginal tamponade combined with abdominal compression for the management of postpartum hemorrhage. J Obstet Gynaecol Can. 2018; 40:561–5.

22. Howard TF, Grobman WA. The relationship between timing of postpartum hemorrhage interventions and adverse outcomes. Am J Obstet Gynecol. 2015; 213:239.e1–3.

23. Shim SR, Kim SJ, Lee J, Rücker G. Network meta-analysis: application and practice using R software. Epidemiol Health. 2019; 41:e2019013.

24. Sterne JA, Hernán MA, Reeves BC, Savović J, Berkman ND, Viswanathan M, et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ. 2016; 355:i4919.

25. Bilandzic A, Fitzpatrick T, Rosella L, Henry D. Risk of bias in systematic reviews of non-randomized studies of adverse cardiovascular effects of thiazolidinediones and cyclooxygenase-2 inhibitors: application of a new Cochrane risk of bias tool. PLoS Med. 2016; 13:e1001987.

26. Zhang YJ, Wang N, Gu ZC, Wei AH, Cheng AN, Fang SS, et al. A network meta-analysis for safety of endothelin receptor antagonists in pulmonary arterial hypertension. Cardiovasc Diagn Ther. 2019; 9:239–49.

27. Gallos ID, Papadopoulou A, Man R, Athanasopoulos N, Tobias A, Price MJ, et al. Uterotonic agents for preventing postpartum haemorrhage: a network meta-analysis. Cochrane Database Syst Rev. 2018; 12:CD011689.

28. Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011; 343:d4002.

29. Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015; 15:58.

30. Yan JY, Zhou ZM, Xu X, Huang XY, Xu RL, Lin SH. Risk factors and surgical interventions associated with primary postpartum haemorrhage unresponsive to first-line therapies. J Obstet Gynaecol. 2014; 34:588–92.

31. Zhao Y, Zhang Y, Li Z. Appropriate second-line therapies for management of severe postpartum hemorrhage. Int J Gynaecol Obstet. 2014; 127:180–2.

32. Dahlke JD, Mendez-Figueroa H, Maggio L, Hauspurg AK, Sperling JD, Chauhan SP, et al. Prevention and management of postpartum hemorrhage: a comparison of 4 national guidelines. Am J Obstet Gynecol. 2015; 213:76.e1–10.

34. Kaya B, Tuten A, Daglar K, Misirlioglu M, Polat M, Yildirim Y, et al. Balloon tamponade for the management of postpartum uterine hemorrhage. J Perinat Med. 2014; 42:745–53.

35. Chen C, Lee SM, Kim JW, Shin JH. Recent update of embolization of postpartum hemorrhage. Korean J Radiol. 2018; 19:585–96.

36. Oyelese Y, Ananth CV. Postpartum hemorrhage: epidemiology, risk factors, and causes. Clin Obstet Gynecol. 2010; 53:147–56.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download