Abstract
We experienced an extremely rare case of proximal epithelioid sarcoma (PES) of the vulva in a 77-year-old woman. After history taking and physical examination, the patient was tentatively diagnosed as having Bartholin’s cyst in the right labium. Based on histopathological and immunohistochemical (IHC) findings, however, a final diagnosis of PES of the vulva was made. After receiving CyberKnife treatment, the patient survived but with recurrent episodes and poor prognosis. In conclusion, our case indicates that patients with PES of the vulva should be appropriately managed with radiotherapy after a differential diagnosis based on histopathological and IHC findings.
Vulvar cancer is the fourth most common gynecologic malignancy, accounting for 5% of all cancers of the female genital tract [1,2]. Of the diverse histological types of vulvar cancer, squamous cell carcinoma is the most common (95%), followed by melanoma, sarcoma, and basalioma [3]. The incidence of primary sarcoma of the vulva is estimated to be approximately 1.5–5% of all vulvar malignancies. Primary sarcoma of the vulva frequently affects the labia majora, Bartholin gland, clitoris, and labium minus [4].
Epithelioid sarcoma (ES) is a malignant soft tissue tumor that can be classified into proximal and distal types. Proximal PES occurs in the trunk and pubic regions, and distal PES occurs in the upper and lower limbs. Of the cases, proximal epithelioid sarcoma (PES) of the vulva is an extremely rare entity, and its occurrence has been described in a paucity of literature [5-7].
We experienced an extremely rare case of PES of the vulva in a 77-year-old woman. Herein, we report our case with a literature review.
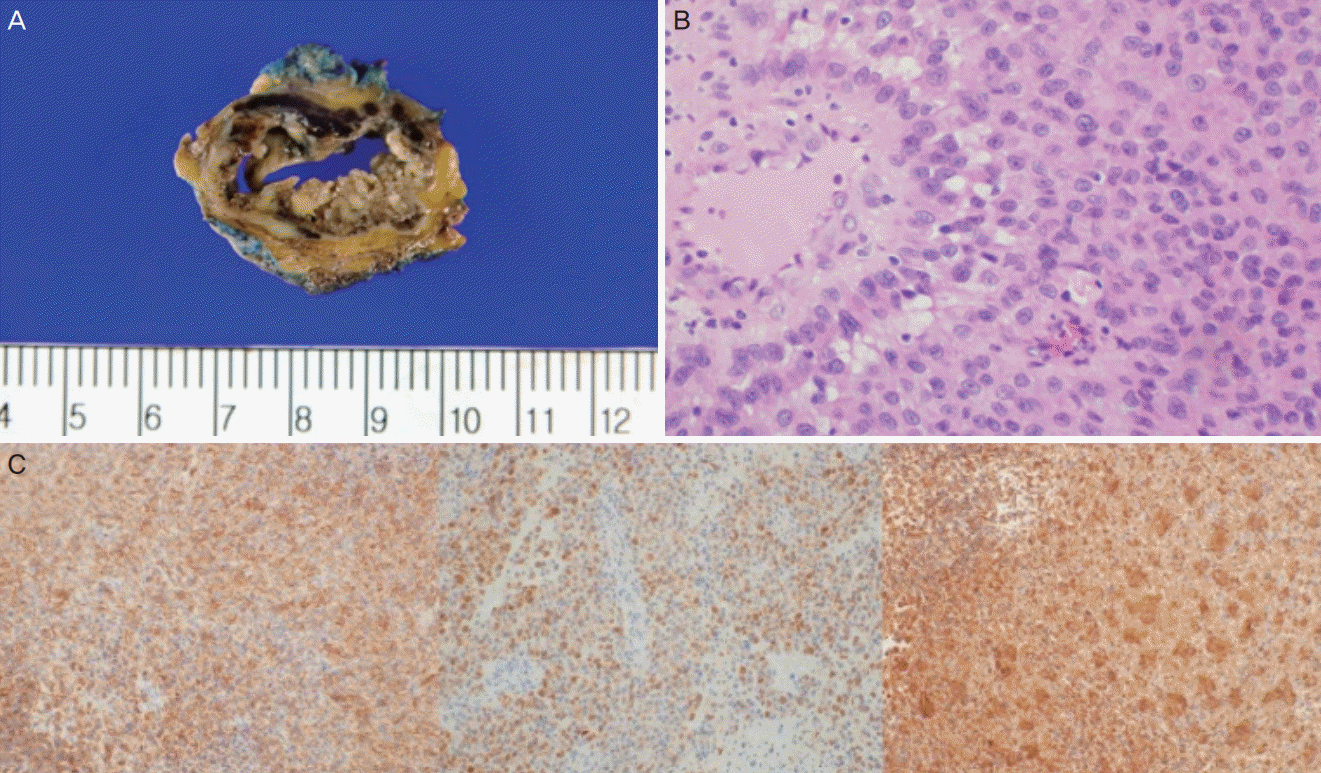
In July 2016, a 77-year-old woman visited our clinic with a chief complaint of asymptomatic mass over the right vulva. On history taking, the patient had no underlying diseases other than hypertension and diabetes mellitus. She had an obstetrical history of 6-0-8(0/8)-4(3/1). Grossly, she had a mass measuring 3×2 cm in size on the skin over the right ower labia, which was suggestive of Bartholin’s cyst in the right labium (Fig. 1A). Therefore, she underwent a Bartholin gland cystectomy. An intraoperative frozen biopsy showed positive findings of malignancy. Moreover, histopathological examinations revealed a poorly differentiated malignant neoplasm with a plump cytoplasm and eccentric nuclei, which was suggestive of PES of the vulva (Fig. 1B). In addition, immunohistochemistry (IHC) was positive for CD34, pancytokeratin (CK-PAN), and vimentin (VT; Fig. 1C).
After the operation, the patient had an enlargement of the right external iliac and inguinal lymph nodes on magnetic resonance imaging (MRI) and positron emission tomography-computed tomography (PET/CT). In August 2016, she underwent a reoperation for excision of the right inguinal lymph node and a laparoscopic excision of the right pelvic lymph node. An intraoperative frozen biopsy confirmed the malignancy in the right pelvic and inguinal lymph nodes. On the basis of the histopathological examination finding, the patient was diagnosed as having a proximal metastatic ES of the vulva. Of the seven lymph nodes, two were involved in the metastasis.
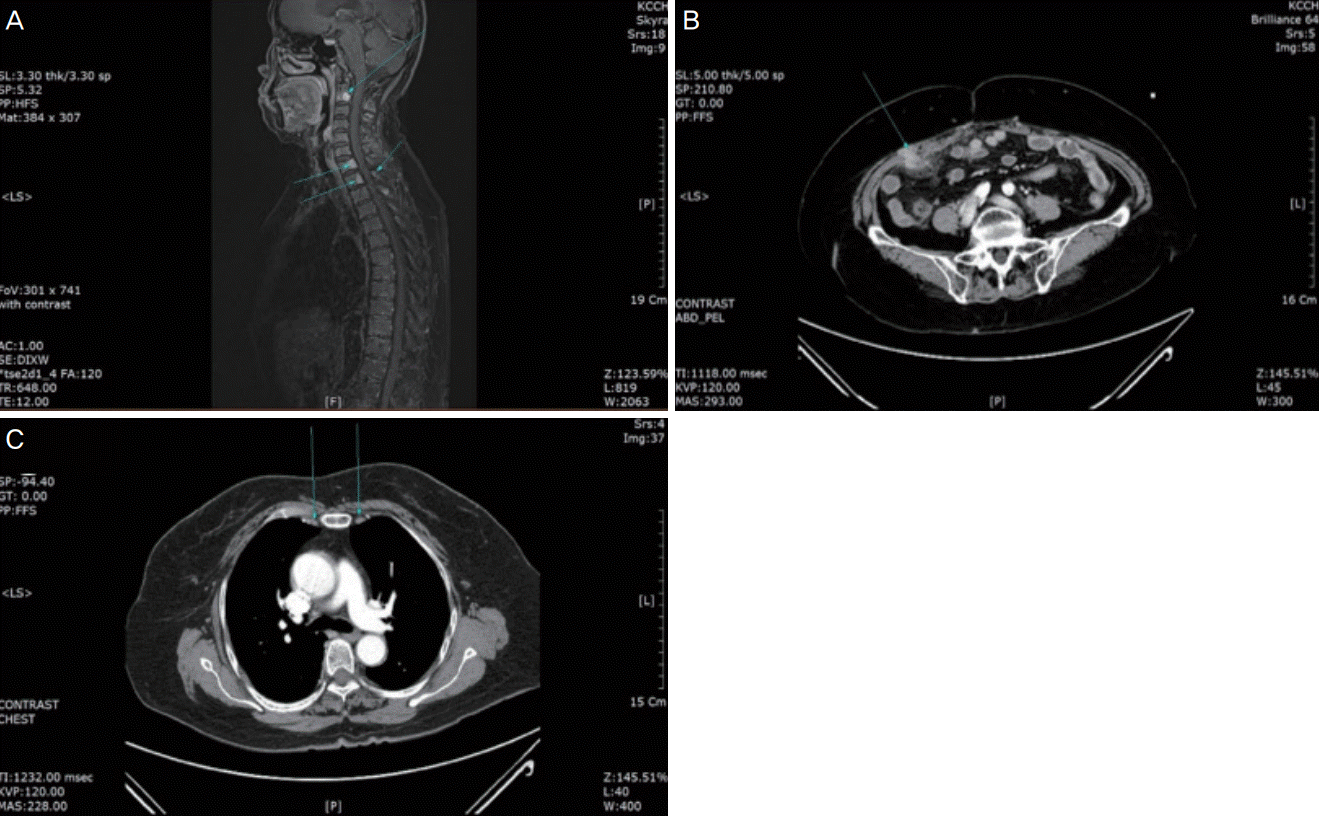
After the postoperative recovery period between September 23 and October 31, 2016, the patient received radiotherapy at a dose of 46.8 Gy/26 Fx to the vulva and pelvic and inguinal lymph nodes. During the course of the radiotherapy, she developed severe skin desquamation. Thus, she completed only 26 of the 28 treatment cycles of radiotherapy. In August 2017, she had a recurrence with a bone metastasis on her chest CT scans at a follow-up visit. Therefore, she underwent further examination with MRI and PET/CT, which revealed multiple bone metastases (Fig. 2, upper panel). Therefore, she additionally received palliative radiotherapy to the C2 spine at a dose of 30 Gy/4 Fx and C5-T2 spine at a dose of 30 Gy/10Fx between September 18 and 29, 2017.
In December 2017, the multiple bone metastases showed increases in size and number on the follow-up MRI scans. Therefore, the patient received CyberKnife treatment to the T8 and T12 spinal cord segments at doses of 36 Gy/4 Fx and 36 Gy/4 Fx, respectively, between January 9 and 18, 2018. Thereafter, the patient was followed up using imaging modalities at 3-month intervals. Eight months later, she underwent abdominal and pelvic CT (APCT) and chest CT, which revealed findings suggestive of multiple peritoneal carcinomatosis (Fig. 2, middle panel). In addition, she presented with lymphadenopathy and caval lymph node metastasis (Fig. 2, lower panel). Therefore, she received CyberKnife treatment to the pericaval lymph node at a dose of 4,800 Gy/4 Fx between October 10 and 26, 2018. Meanwhile, she complained of dyspnea, for which she underwent chest posteroanterior radiography, which revealed a small amount of pleural effusion in both lungs. The fluid cytology findings were negative for malignancy, so the patient received only conservative management with chest tube insertion for chest pleural effusion drainage. Subsequently, on APCT and chest CT, the peritoneal carcinomatosis and lymphadenopathy had aggravated, accompanied by a new bone metastasis. The patient was therefore recommended to undergo chemotherapy, but she and her caregivers refused the treatment because of her old age and poor systemic condition. For >3 years after disease onset, she survived while receiving supportive care. The patient was followed up until May 23, 2019.
ES is a soft tissue malignancy. The first case of PES of the vulva was described by Pier et al. in 1972 [8]. It often affects the labia majora of young women, and its differential diagnoses include Bartholin’s cyst, lipoma, and genital warts [7,9]. Its benign appearance as an asymptomatic subcutaneous nodule poses diagnostic and therapeutic dilemmas for clinicians [8-10]. On histopathological examination, PES of the vulva can be observed as plump spindle cells or large polygonal cells with a deeply acidophilic cytoplasm, which resemble epithelioid or squamous cells. Therefore, it can be diagnosed using IHC [6]. Our case showed high IHC positivity rates for CD34, CK-PAN, and VT, which is in agreement with the results of the previous studies in this series [11,12].
No treatment guidelines have been established for PES of the vulva [5]. However, surgical excision with a wide margin of >2 cm remains the mainstay of treatment. A strong correlation was found between inadequate margins and increased risk of local recurrence. Poor prognosis is associated with the presence of lymphatic metastasis. Adjuvant radiotherapy is recommended for high-grade tumors or cases with inadequate surgical margins [13]. We did not perform chemotherapy for our case, in accordance with previous studies that showed that the treatment effects of postoperative chemotherapy are still controversial [7,11-14]. Argenta et al. [13] performed adjuvant chemotherapy for 7 of 31 patients with vulva ES or malignant rhabdoid tumor and reported that of the 7 patients, 3 were disease-free at 8, 11, and 21 months but the remaining 4 died of disseminated disease within 8 months of diagnosis.
PES of the vulva is an extremely rare entity, with only 29 cases described in the English literature. According to the previous studies in this series, the median age of onset was 36.4 years; most of the cases occurred during the early postmenopausal period except, while some cases occurred in young women [7].
In the present case, active radiotherapy using the CyberKnife treatment for the metastatic site was effective in prolonging survival. A poor prognosis is indicated in PES of the vulva. Patients with PES of the vulva are vulnerable to local recurrence despite the presence of negative surgical margins, and up to 60% of them experience a distant metastasis [15-18]. According to Hasegawa et al. [16], the tumor-related mortality rate was 65% in 20 cases of PES of the vulva, which was lower than the metastasis rate (75%). Moreover, Ulbright et al. [19] reported 100% mortality due to distant metastasis in patients with local recurrence.
In conclusion, our case indicates that patients with PES of the vulva should be appropriately managed with radiotherapy after a differential diagnosis on the basis of histopathological and IHC findings.
Notes
References
3. Günther V, Alkatout I, Lez C, Altarac S, Fures R, Cupic H, et al. Malignant melanoma of the urethra: a rare histologic subdivision of vulvar cancer with a poor prognosis. Case Rep Obstet Gynecol. 2012; 2012:385175.

4. Ulutin HC, Zellars RC, Frassica D. Soft tissue sarcoma of the vulva: a clinical study. Int J Gynecol Cancer. 2003; 13:528–31.

5. Iavazzo C, Gkegkes ID, Vrachnis N. Dilemmas in the management of patients with vulval epithelioid sarcoma: a literature review. Eur J Obstet Gynecol Reprod Biol. 2014; 176:1–4.

6. Kim HJ, Kim MH, Kwon J, Kim JY, Park K, Ro JY. Proximal-type epithelioid sarcoma of the vulva with INI1 diagnostic utility. Ann Diagn Pathol. 2012; 16:411–5.

7. Patrizi L, Corrado G, Saltari M, Perracchio L, Scelzo C, Piccione E, et al. Vulvar “proximal-type” epithelioid sarcoma: report of a case and review of the literature. Diagn Pathol. 2013; 8:122.

8. Piver MS, Tsukada Y, Barlow J. Epithelioid sarcoma of the vulva. Obstet Gynecol. 1972; 40:839–42.
9. Rodrigues AI, Lopes HI, Lima O, Marta S. Proximal-type epithelioid sarcoma-unusual presentation: unilateral vulvar mass. BMJ Case Rep. 2015; 2015:bcr2014208488.

10. Moore RG, Steinhoff MM, Granai CO, DeMars LR. Vulvar epithelioid sarcoma in pregnancy. Gynecol Oncol. 2002; 85:218–22.

11. Rego JL, Cintra GF, Netto AK, Abrahão-Machado LF, Tsunoda A. Extremely rare case of vulvar myxoid epithelioid sarcoma. Case Rep Obstet Gynecol. 2015; 2015:971217.

12. Kim JH, Choi YS, Lee TS. A case of epithelioid sarcoma arising in the vulva. J Gynecol Oncol. 2008; 19:202–4.

13. Argenta PA, Thomas S, Chura JC. Proximal-type epithelioid sarcoma vs. malignant rhabdoid tumor of the vulva: a case report, review of the literature, and an argument for consolidation. Gynecol Oncol. 2007; 107:130–5.

14. Coates SJ, Ogunrinade O, Lee HJ, Desman G. Epidermotropic metastatic epithelioid sarcoma: a potential diagnostic pitfall. J Cutan Pathol. 2014; 41:672–6.

15. Chase DR, Enzinger FM. Epithelioid sarcoma. Diagnosis, prognostic indicators, and treatment. Am J Surg Pathol. 1985; 9:241–63.
16. Hasegawa T, Matsuno Y, Shimoda T, Umeda T, Yokoyama R, Hirohashi S. Proximal-type epithelioid sarcoma: a clinicopathologic study of 20 cases. Mod Pathol. 2001; 14:655–63.

17. Evans HL, Baer SC. Epithelioid sarcoma: a clinicopathologic and prognostic study of 26 cases. Semin Diagn Pathol. 1993; 10:286–91.
Fig. 1.
Gross, histopathological, and immunohistochemical findings. (A) Shown is the gross finding of a mass of 3×2 cm in size in the skin over the right lower labia, which is suggestive of Bartholin’s cyst in the right labium. (B) The histopathological examination result shows a poorly differentiated malignant neoplasm with a plump cytoplasm and eccentric nuclei. (C) From left to right are the positive immunohistochemical findings for CD34, pan-cytokeratin, and vimentin.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download