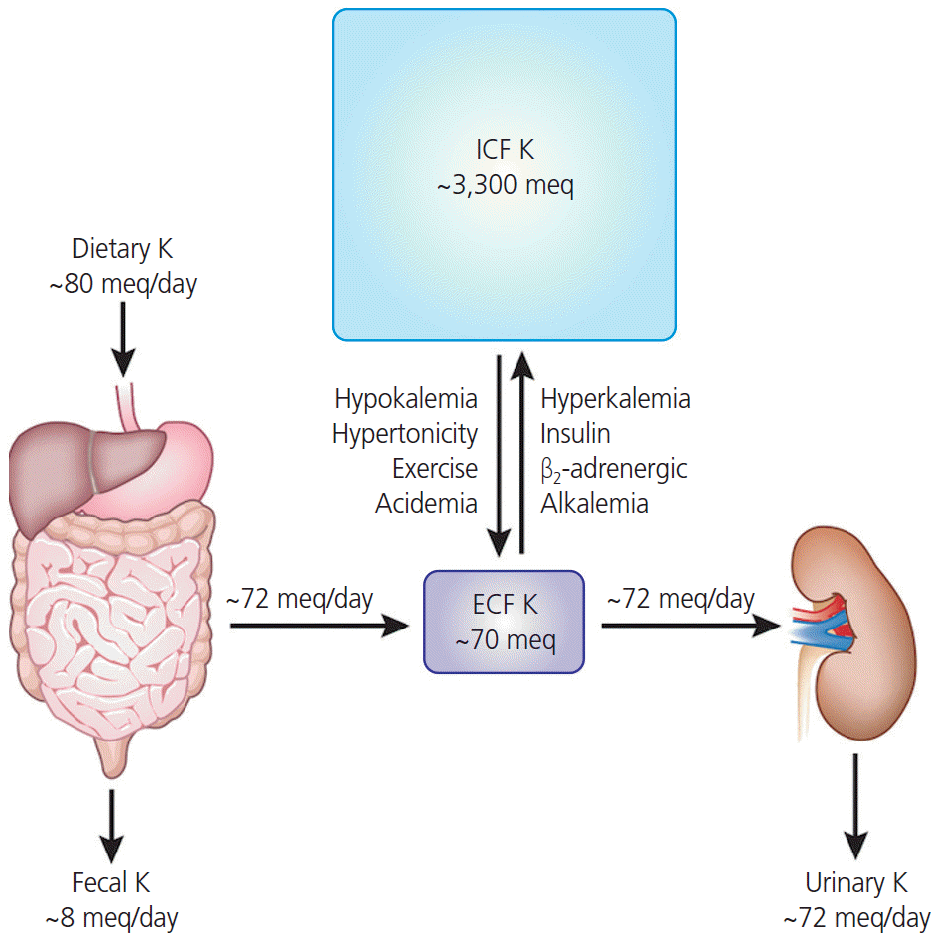
1. Aronson PS, Giebisch G. Effects of pH on potassium: new explanations for old observations. J Am Soc Nephrol. 2011; 22:1981–9.

2. Gumz ML, Rabinowitz L, Wingo CS. An integrated view of potassium homeostasis. N Engl J Med. 2015; 373:60–72.

3. Palmer BF. Regulation of potassium homeostasis. Clin J Am Soc Nephrol. 2015; 10:1050–60.

4. DuBose TD Jr. Regulation of potassium homeostasis in CKD. Adv Chronic Kidney Dis. 2017; 24:305–14.

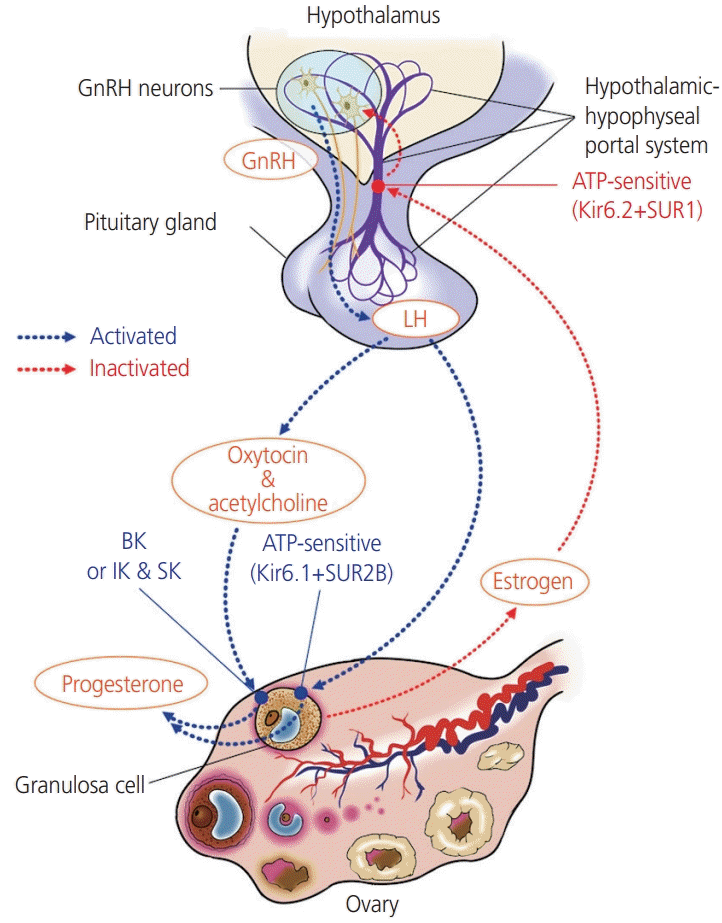
5. Kunz L, Richter JS, Mayerhofer A. The adenosine 5'-triphosphate-sensitive potassium channel in endocrine cells of the human ovary: role in membrane potential generation and steroidogenesis. J Clin Endocrinol Metab. 2006; 91:1950–5.

6. Comes N, Serrano-Albarrás A, Capera J, Serrano-Novillo C, Condom E, Ramón Y Cajal S, et al. Involvement of potassium channels in the progression of cancer to a more malignant phenotype. Biochim Biophys Acta. 2015; 1848 10 Pt B:2477–92.

7. Burg ED, Remillard CV, Yuan JX. K+ channels in apoptosis. J Membr Biol. 2006; 209:3–20.

8. Hebert SC. An ATP-regulated, inwardly rectifying potassium channel from rat kidney (ROMK). Kidney Int. 1995; 48:1010–6.

9. Tian C, Zhu R, Zhu L, Qiu T, Cao Z, Kang T. Potassium channels: structures, diseases, and modulators. Chem Biol Drug Des. 2014; 83:1–26.

10. Kuang Q, Purhonen P, Hebert H. Structure of potassium channels. Cell Mol Life Sci. 2015; 72:3677–93.

11. Wareing M, Greenwood SL. Review: potassium channels in the human fetoplacental vasculature. Placenta. 2011; 32 Suppl 2:S203–6.

12. Brainard AM, Korovkina VP, England SK. Potassium channels and uterine function. Semin Cell Dev Biol. 2007; 18:332–9.

13. Díaz P, Sibley CP, Greenwood SL. Oxygen-sensitive K+ channels modulate human chorionic gonadotropin secretion from human placental trophoblast. PLoS One. 2016; 11:e0149021.

14. Hummitzsch K, Anderson RA, Wilhelm D, Wu J, Telfer EE, Russell DL, et al. Stem cells, progenitor cells, and lineage decisions in the ovary. Endocr Rev. 2015; 36:65–91.

15. Engel S, Klusmann H, Ditzen B, Knaevelsrud C, Schumacher S. Menstrual cycle-related fluctuations in oxytocin concentrations: a systematic review and meta-analysis. Front Neuroendocrinol. 2019; 52:144–55.

16. Kunz L, Thalhammer A, Berg FD, Berg U, Duffy DM, Stouffer RL, et al. Ca2+-activated, large conductance K+ channel in the ovary: identification, characterization, and functional involvement in steroidogenesis. J Clin Endocrinol Metab. 2002; 87:5566–74.
17. Mayerhofer A, Föhr KJ, Sterzik K, Gratzl M. Carbachol increases intracellular free calcium concentrations in human granulosa-lutein cells. J Endocrinol. 1992; 135:153–9.

18. Mayerhofer A, Sterzik K, Link H, Wiemann M, Gratzl M. Effect of oxytocin on free intracellular Ca2+ levels and progesterone release by human granulosa-lutein cells. J Clin Endocrinol Metab. 1993; 77:1209–14.

19. Copland JA, Zlatnik MG, Ives KL, Soloff MS. Oxytocin receptor regulation and action in a human granulosalutein cell line. Biol Reprod. 2002; 66:1230–6.
20. Traut MH, Berg D, Berg U, Mayerhofer A, Kunz L. Identification and characterization of Ca2+-activated K+ channels in granulosa cells of the human ovary. Reprod Biol Endocrinol. 2009; 7:28.

21. Gross SA, Newton JM, Hughes FM Jr. Decreased intracellular potassium levels underlie increased progesterone synthesis during ovarian follicular atresia. Biol Reprod. 2001; 64:1755–60.
22. Fujita R, Kimura S, Kawasaki S, Watanabe S, Watanabe N, Hirano H, et al. Electrophysiological and pharmacological characterization of the K(ATP) channel involved in the K+-current responses to FSH and adenosine in the follicular cells of Xenopus oocyte. J Physiol Sci. 2007; 57:51–61.

23. Chien EK, Zhang Y, Furuta H, Hara M. Expression of adenosine triphosphate-sensitive potassium channel subunits in female rat reproductive tissues: overlapping distribution of messenger ribonucleic acid for weak inwardly rectifying potassium channel subunit 6.1 and sulfonylurea-binding regulatory subunit 2. Am J Obstet Gynecol. 1999; 180:1121–6.

24. Agoston A, Kunz L, Krieger A, Mayerhofer A. Two types of calcium channels in human ovarian endocrine cells: involvement in steroidogenesis. J Clin Endocrinol Metab. 2004; 89:4503–12.

25. Kunz L, Rämsch R, Krieger A, Young KA, Dissen GA, Stouffer RL, et al. Voltage-dependent K+ channel acts as sex steroid sensor in endocrine cells of the human ovary. J Cell Physiol. 2006; 206:167–74.
26. Zhang C, Bosch MA, Levine JE, Rønnekleiv OK, Kelly MJ. Gonadotropin-releasing hormone neurons express K(ATP) channels that are regulated by estrogen and responsive to glucose and metabolic inhibition. J Neurosci. 2007; 27:10153–64.

27. Huang W, Acosta-Martínez M, Levine JE. Ovarian steroids stimulate adenosine triphosphate-sensitive potassium (KATP) channel subunit gene expression and confer responsiveness of the gonadotropin-releasing hormone pulse generator to KATP channel modulation. Endocrinology. 2008; 149:2423–32.

28. Ashcroft FM, Gribble FM. New windows on the mechanism of action of K(ATP) channel openers. Trends Pharmacol Sci. 2000; 21:439–45.
29. Kelly MJ, Rønnekleiv OK, Ibrahim N, Lagrange AH, Wagner EJ. Estrogen modulation of K(+) channel activity in hypothalamic neurons involved in the control of the reproductive axis. Steroids. 2002; 67:447–56.

30. Pielecka-Fortuna J, DeFazio RA, Moenter SM. Voltage-gated potassium currents are targets of diurnal changes in estradiol feedback regulation and kisspeptin action on gonadotropin-releasing hormone neurons in mice. Biol Reprod. 2011; 85:987–95.
31. Norberg R, Campbell R, Suter KJ. Ion channels and information processing in GnRH neuron dendrites. Channels (Austin). 2013; 7:135–45.

32. Vastagh C, Solymosi N, Farkas I, Liposits Z. Proestrus differentially regulates expression of ion channel and calcium homeostasis genes in GnRH neurons of mice. Front Mol Neurosci. 2019; 12:137.

33. Yin Z, Li Y, He W, Li D, Li H, Yang Y, et al. Progesterone inhibits contraction and increases TREK-1 potassium channel expression in late pregnant rat uterus. Oncotarget. 2017; 9:651–61.

34. Yin Z, Sada AA, Reslan OM, Narula N, Khalil RA. Increased MMPs expression and decreased contraction in the rat myometrium during pregnancy and in response to prolonged stretch and sex hormones. Am J Physiol Endocrinol Metab. 2012; 303:E55–70.

35. Aaronson PI, Sarwar U, Gin S, Rockenbauch U, Connolly M, Tillet A, et al. A role for voltage-gated, but not Ca2+-activated, K+ channels in regulating spontaneous contractile activity in myometrium from virgin and pregnant rats. Br J Pharmacol. 2006; 147:815–24.

36. Wang SY, Yoshino M, Sui JL, Wakui M, Kao PN, Kao CY. Potassium currents in freshly dissociated uterine myocytes from non-pregnant and late-pregnant rats. J Gen Physiol. 1998; 112:737–56.

37. Benkusky NA, Fergus DJ, Zucchero TM, England SK. Regulation of the Ca2+-sensitive domains of the maxi-K channel in the mouse myometrium during gestation. J Biol Chem. 2000; 275:27712–9.

38. Pérez G, Toro L. Differential modulation of large-conductance KCa channels by PKA in pregnant and non-pregnant myometrium. Am J Physiol. 1994; 266:C1459–63.
39. Benkusky NA, Korovkina VP, Brainard AM, England SK. Myometrial maxi-K channel beta1 subunit modulation during pregnancy and after 17beta-estradiol stimulation. FEBS Lett. 2002; 524:97–102.
40. Okawa T, Longo M, Vedernikov YP, Chwalisz K, Saade GR, Garfield RE. Role of nucleotide cyclases in the inhibition of pregnant rat uterine contractions by the openers of potassium channels. Am J Obstet Gynecol. 2000; 182:913–8.

41. Sadlonova V, Franova S, Dokus K, Janicek F, Visnovsky J, Sadlonova J. Participation of BKCa2+ and KATP potassium ion channels in the contractility of human term pregnant myometrium in in vitro conditions. J Obstet Gynaecol Res. 2011; 37:215–21.

42. Ferreira JJ, Butler A, Stewart R, Gonzalez-Cota AL, Lybaert P, Amazu C, et al. Oxytocin can regulate myometrial smooth muscle excitability by inhibiting the Na+ -activated K+ channel, Slo2.1. J Physiol. 2019; 597:137–49.
43. Curley M, Cairns MT, Friel AM, McMeel OM, Morrison JJ, Smith TJ. Expression of mRNA transcripts for ATP-sensitive potassium channels in human myometrium. Mol Hum Reprod. 2002; 8:941–5.

44. Smith RC, McClure MC, Smith MA, Abel PW, Bradley ME. The role of voltage-gated potassium channels in the regulation of mouse uterine contractility. Reprod Biol Endocrinol. 2007; 5:41.

45. Modzelewska B, Kostrzewska A, Sipowicz M, Kleszczewski T, Batra S. Apamin inhibits NO-induced relaxation of the spontaneous contractile activity of the myometrium from non-pregnant women. Reprod Biol Endocrinol. 2003; 1:8.
46. Noble K, Floyd R, Shmygol A, Shmygol A, Mobasheri A, Wray S. Distribution, expression and functional effects of small conductance Ca-activated potassium (SK) channels in rat myometrium. Cell Calcium. 2010; 47:47–54.

47. Rosenbaum ST, Larsen T, Joergensen JC, Bouchelouche PN. Relaxant effect of a novel calcium-activated potassium channel modulator on human myometrial spontaneous contractility in vitro. Acta Physiol (Oxf). 2012; 205:247–54.

48. Jensen BS, Hertz M, Christophersen P, Madsen LS. The Ca2+-activated K+ channel of intermediate conductance:a possible target for immune suppression. Expert Opin Ther Targets. 2002; 6:623–36.
49. Bond CT, Sprengel R, Bissonnette JM, Kaufmann WA, Pribnow D, Neelands T, et al. Respiration and parturition affected by conditional overexpression of the Ca2+-activated K+ channel subunit, SK3. Science. 2000; 289:1942–6.

50. Rosenbaum ST, Svalø J, Nielsen K, Larsen T, Jørgensen JC, Bouchelouche P. Immunolocalization and expression of small-conductance calcium-activated potassium channels in human myometrium. J Cell Mol Med. 2012; 16:3001–8.

51. Rahbek M, Nazemi S, Odum L, Gupta S, Poulsen SS, Hay-Schmidt A, et al. Expression of the small conductance Ca2+-activated potassium channel subtype 3 (SK3) in rat uterus after stimulation with 17β-estradiol. PLoS One. 2014; 9:e87652.

52. Greenwood IA, Tribe RM. Kv7 and Kv11 channels in myometrial regulation. Exp Physiol. 2014; 99:503–9.

53. Gutman GA, Chandy KG, Adelman JP, Aiyar J, Bayliss DA, Clapham DE, et al. International Union of Pharmacology. XLI. Compendium of voltage-gated ion channels: potassium channels. Pharmacol Rev. 2003; 55:583–6.

54. McCallum LA, Pierce SL, England SK, Greenwood IA, Tribe RM. The contribution of Kv7 channels to pregnant mouse and human myometrial contractility. J Cell Mol Med. 2011; 15:577–86.

55. Greenwood IA, Yeung SY, Tribe RM, Ohya S. Loss of functional K+ channels encoded by ether-à-go-go-related genes in mouse myometrium prior to labour onset. J Physiol. 2009; 587:2313–26.
56. Heyman NS, Cowles CL, Barnett SD, Wu YY, Cullison C, Singer CA, et al. TREK-1 currents in smooth muscle cells from pregnant human myometrium. Am J Physiol Cell Physiol. 2013; 305:C632–42.

57. Buxton IL, Heyman N, Wu YY, Barnett S, Ulrich C. A role of stretch-activated potassium currents in the regulation of uterine smooth muscle contraction. Acta Pharmacol Sin. 2011; 32:758–64.

58. Yin Z, He W, Li Y, Li D, Li H, Yang Y, et al. Adaptive reduction of human myometrium contractile activity in response to prolonged uterine stretch during term and twin pregnancy. Role of TREK-1 channel. Biochem Pharmacol. 2018; 152:252–63.

59. Weedon-Fekjær MS, Taskén K. Review: Spatiotemporal dynamics of hCG/cAMP signaling and regulation of placental function. Placenta. 2012; 33 Suppl:S87–91.

60. Brereton MF, Wareing M, Jones RL, Greenwood SL. Characterisation of K+ channels in human fetoplacental vascular smooth muscle cells. PLoS One. 2013; 8:e57451.

61. Wareing M, Bai X, Seghier F, Turner CM, Greenwood SL, Baker PN, et al. Expression and function of potassium channels in the human placental vasculature. Am J Physiol Regul Integr Comp Physiol. 2006; 291:R437–46.

62. Li FF, He MZ, Xie Y, Wu YY, Yang MT, Fan Y, et al. Involvement of dysregulated IKCa and SKCa channels in preeclampsia. Placenta. 2017; 58:9–16.

63. Kang KT. Endothelium-derived relaxing factors of small resistance arteries in hypertension. Toxicol Res. 2014; 30:141–8.

64. Choi SK, Hwang JY, Lee J, Na SH, Ha JG, Lee HA, et al. Gene Expression of Heme Oxygenase-1 and Nitric Oxide Synthase on Trophoblast of Preeclampsia. Korean J Obstet Gynecol. 2011; 54:341–8.
65. Köhler R, Hoyer J. The endothelium-derived hyperpolarizing factor: insights from genetic animal models. Kidney Int. 2007; 72:145–50.

66. Zhu R, Hu XQ, Xiao D, Yang S, Wilson SM, Longo LD, et al. Chronic hypoxia inhibits pregnancy-induced upregulation of SKCa channel expression and function in uterine arteries. Hypertension. 2013; 62:367–74.
67. Yang Q, Huang JH, Man YB, Yao XQ, He GW. Use of intermediate/small conductance calcium-activated potassium-channel activator for endothelial protection. J Thorac Cardiovasc Surg. 2011; 141:501–10.

68. Milan R, Flores-Herrera O, Espinosa-Garcia MT, OlveraSanchez S, Martinez F. Contribution of potassium in human placental steroidogenesis. Placenta. 2010; 31:860–6.





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download