Introduction
Hysteroscopy is a minimally invasive intervention that can be used to diagnose and treat many intrauterine and endocervical problems. Diagnostic and operative hysteroscopy have become standard in gynecologic practice. In Sweden, approximately 5,000 hysteroscopy operations are performed every year. At Sahlgrenska University Hospital Östra, approximately 160 hysteroscopy operations are performed every year [
1].
Pain is sometimes a problem after hysteroscopy [
2] and conservative treatment with opioids often gives adequate pain relief [
3]. However, opioids have serious side effects, such as sedation, nausea and respiratory depression [
4]. Hence, the patient needs surveillance, resulting in a longer time spent in the Post-Anesthesia Care Unit (PACU) after surgery.
Furthermore, there is a serious concern regarding “the opioid epidemic” in the United States [
5], where society faces an escalating problem of the use of opioids after surgery, which has led to opioid addiction and sometimes abuse in some patients. Prolonged opioid use after surgery is a common and previously underestimated problem [
6]. The risk of previous opioid naive patients to end up in long-term opioid use is estimated at approximately 6% after both major and minor interventions [
6], indicating that long-term opioid use might be considered as a common complication after surgery. Hence, there is an increased interest in the use of non-opioid alternatives for the treatment of postoperative pain [
7].
Transcutaneous electrical nerve stimulation (TENS) is a pain treatment that delivers an electrical current through the skin. The exact mechanism of action of TENS treatment is still unknown. However, the effects of peripheral nerve stimulation has traditionally been linked to the activation of afferent nerve fibers (Aβ-fibers) modulating Aδ and C-fibers in the spinal cord, which is compatible with the gate control theory of pain [
8]. Release of endogenous endorphins following high-frequency TENS application has also been suggested as a mechanism of action [
9,
10]. Furthermore, the diffuse noxious inhibitory controls theory (DNIC) refers to an endogenous pain modulatory pathway, which has often been described as “pain inhibiting pain through continuous pain” by noxious or intense cutaneous stimulation, such as TENS [
11].
TENS has been shown to be effective for treatment of a variety of gynecological conditions such as dysmenorrhea, pain after endometrial biopsy, surgical abortion, and gynecological laparoscopy, as well as labor pain [
12-
17]. In addition, previous studies from our center indicate that TENS treatment for postoperative pain results in a shorter time in the PACU [
16,
17].
Studies by De Angelis et al. [
18] and Lisón et al. [
19] have indicated that TENS treatment is effective for intraoperative pain relief during hysteroscopy. However, to our knowledge, there is limited data concerning the effects of TENS for postoperative pain treatment after hysteroscopy.
Hence, the aim of the present randomized control study was to compare the time spent in the PACU after surgery as well as the postoperative pain relieving effects of high-frequency, high-intensity TENS and pharmacological treatment compared to intravenous (IV) opioids in patients undergoing hysteroscopy.
Our hypothesis was that patients receiving TENS for pain relief after undergoing hysteroscopy would spend a shorter amount of time in the PACU than those treated with pharmacological IV opioids.
Go to :

Discussion
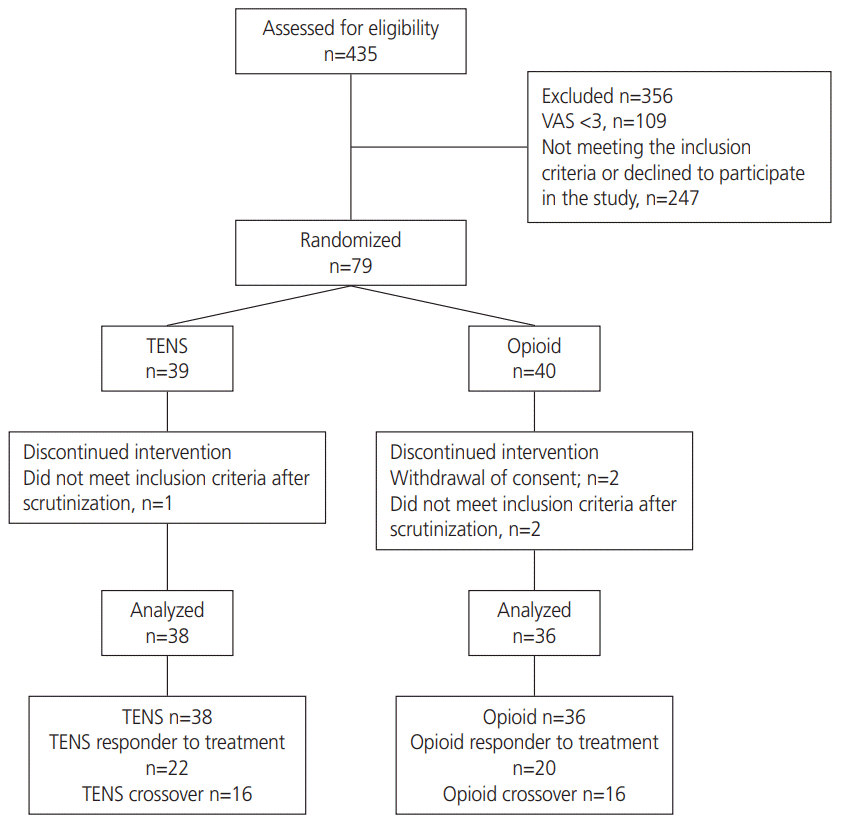
The results from this randomized controlled study indicate that both treatment with high-intensity, high-frequency stimulation using TENS and pharmacological treatment with IV opioids are effective for postoperative pain relief after gynecologic hysteroscopy. This is consistent with previous studies demonstrating the effectiveness of TENS for postoperative pain relief [
16,
17]. However, there was no difference between the groups with regards to time spent in the PACU after surgery. When only responders to treatment were compared, both groups reported similar pain relief, but the TENS group spent a shorter time in the PACU.
If the patient responds to the TENS treatment, the patient reports instant pain relief with no need for monitoring of adverse side-effects such as sedation, nausea and respiratory depression after treatment. This is a clinically relevant advantage with the TENS treatment modality, since it only takes two minutes to assess if the patient responds to it or not. In contrast, it takes approximately 20 minutes to evaluate the effects of pharmacological treatment with IV opioid [
22]. Thus, pharmacological treatment with IV opioids is delayed by at most a few minutes if the patient does not respond to TENS, making TENS a more preferable choice for initial pain treatment in the PACU.
Chronic pain after surgery in the lower part of the abdomen is a frequent complication, with a study showing that 5–32% of the patients develop chronic pain after hysterectomy [
23]. Thus, it is important to achieve optimal postoperative pain treatment to prevent development of chronic pain. More than 2/3 of the patients reported moderate or severe pain upon arrival in the PACU. Thus, obtaining adequate pain relief was difficult for many patients, resulting in a high number of crossovers in more than 40% of the patients (n=32) wherein they received both pharmacological treatment with IV opioid and TENS to obtain sufficient pain relief (VAS, <3). The crossover group did not differ from the responders to treatment with regards to pain intensity or previous chronic pain. Nevertheless, opioid consumption was significantly lower in the TENS group. Thus, using TENS as the first line of pain relief may reduce the need for postoperative opioids.
Previously, other methods for peri- and postoperative pain relief such as para-cervical blockade has been tested after hysteroscopy. However, these have limited effects on postoperative pain after uterus intervention [
24]. Furthermore paracervical blockade entails a risk of inducing bradycardia and hypotension, which is likely a result of accidental intravascular injection [
25]. Thus, TENS seems to be a more effective method for postoperative pain relief after hysteroscopy with the benefit of being completely reversible within seconds and with limited side effects.
The importance of properly managing postoperative pain treatment is well known partly because of the risk of developing chronic pain after surgery due to insufficient pain treatment [
26]. Furthermore, it is important to reduce the suffering of patients and minimize any complications due to inadequate pain treatment, such as impaired respiration and immobilization.
In the pursuit of reducing or even replacing opioids, a less harmful alternative for the patient such as TENS might be considered a suitable choice due to the minimal frequency of side effects associated with short-term TENS treatment. Based on the results of this present study and the possible serious short- and long-term complications of opioid treatment, TENS may to preferable as a first line of pain relief in patients undergoing hysteroscopy reporting a postoperative pain VAS score of ≥3.
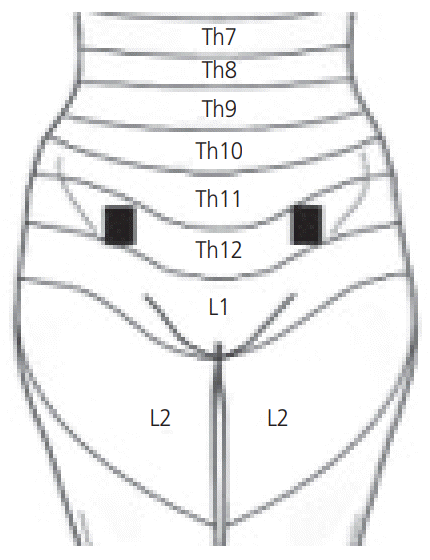
In the present study, high-frequency (80 Hz), high-intensity (40–60 mA) TENS stimulation for 60 seconds was used, and if needed, was repeated once. This is the same treatment model used for primary dysmenorrhea, surgical abortion, gynecological laparoscopy and angina pectoris [
13,
16,
17,
27]. It is important to achieve a strong stimulation level (at least 40 mA). By gradually increasing stimulation intensity, most patients tolerated the treatment, which is in line with previous studies [
16,
17]. Stimulation intensity seems to be crucial for the pain-relieving effect. Results from a study by Moran et al. [
28] indicate that strong non-painful intensities seem more efficient than mild intensity stimulation. Furthermore, for optimum pain relief, it seems important to adjust the position of the electrodes so that the paresthesia over the abdomen covers the area in which the patient localized the abdominal pain. Before the start of the treatment, skin sensation should be assessed, since TENS should not be used if there is impaired sensation. There were no reported side effects of TENS treatment in the study except for intolerable intensities of stimulation for some patients. However, the stimulation lasted for only 60 seconds and all the women in the TENS group were informed that this short period of (uncomfortable) high-intensity TENS was needed to obtain postoperative pain relief. To acclimatize the patients, the stimulation intensity was increased gradually to obtain a high-intensity stimulation level. An additional advantage of TENS treatment is that there is no need to monitor the patients after the treatment. This is in contrast to pharmacological treatment with IV opioids, wherein patients need to be assessed due to the risk of respiratory depression associated with opioids. The clinical routine for TENS treatment at our center has been previously described in detail [
16,
17].
In addition to the pain-relieving effect of TENS and opioids, the cost of treatment needs to be considered. The TENS device used in the present study costs 121 Euros. At our center, costs for consumable materials, i.e., the reusable electrodes and electrode gel, amounted to less than approximately 20 Euros for the study patients, i.e., 0.37 Euro per patient. In comparison, one ampoule of 10 milligrams per milliliter morphine costs 0.31 Euros. In the present study, 1 to 2 ampoules of morphine were used per patient. However, the TENS device can be used for several daily treatments for up to 10 years. Furthermore, assistant nurses can administrate the TENS treatment, whereas morphine can only be administrated by nurses (or physicians) and the patient needs to be monitored for at least 30 minutes due to side-effects, especially respiratory depression. Hence, the nurses’ competence and time can be used for other specific tasks when using TENS for postoperative pain treatment. Furthermore, if the patient responded to TENS treatment, their time spent in the PACU was reduced by 22 minutes, which has an impact on the overall cost for the stay in PACU and which enables them to perform more operations per day.
First, the surgical indication for hysteroscopy affects the time and difficulty of the surgery as well as the quantity of distending media, among others, which may in turn affect postoperative pain. In the present study, there was no difference with regards to indication for hysteroscopy and time of surgery between the groups. Hence, the peri-operative conditions seem to not have affected the patients’ reported postoperative pain at the PACU before treatment.
Second, it was not possible to have a control group untreated for postoperative pain for ethical reasons. Hence, pharmacological treatment with IV opioids and TENS treatment were compared with each other. It would have been a good addition to have blinded the patients and investigators to their respective treatment allocations. However, due to technical and clinical logistic reasons, it was not possible to blind the investigators, and most importantly the patients, to the treatment allocation. This might have affected the outcome. Nevertheless, it should be emphasized that the patients reported their own experiences pain intensities using the VAS scale (i.e., the nurse did not estimate or influence the patient’s report).
Third, the large number of crossovers might have affected the results.
Fourth, according to the power calculation, 100 patients should be included to demonstrate a possible statistical difference between the groups. However, due to reorganization in the hospital resulting in allocation of the patient groups to a different hospital, only 79 patients were randomized for the present study.
In general, results from clinical trials will be stronger with the same results from other independent groups. Hence, further randomized controlled studies are warranted to confirm the pain-relieving effect of TENS on postoperative pain and its effects on opioid consumption.
TENS and short-term pharmacological treatment with IV opioids are both effective treatments for pain relief after gynecologic hysteroscopy, with more than one third of patients reporting complete pain relief. However, many patients seem to need a combination of both treatments to obtain adequate pain relief. Thus, further studies are warranted to further characterize this group of patients.
TENS may be preferable as a first line of treatment since it is associated with a shorter time spent in the PACU and a faster onset of pain relief if the patient responds to the treatment compared to IV opioids. In addition, the effects of TENS treatment can be assessed in a few minutes, which allows for a prompt shift to pharmacological treatment with IV opioids if the patient does not respond to TENS treatment. Thus, TENS as a first-line pain relief method is an option to reduce the need for postoperative opioids.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download