Introduction
Materials and methods
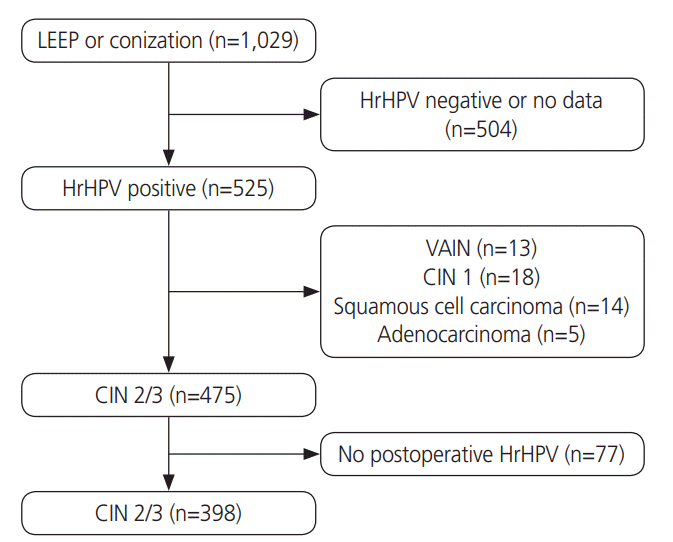
1. Study population and inclusion criteria
2. Human papillomavirus (HPV) test with Anyplex™ II
3. Human papillomavirus (HPV) persistence and genotype-specific persistence
4. Statistical analysis
Results
Table 1.
| Characteristics |
Postop hrHPV persistence (until 12 mon) |
P-value | ||
|---|---|---|---|---|
| Negative (n=244, 61.3) | Positive (n=154, 38.7) | |||
| Age (yr) | 38.37±10.57 | 41.96±12.63 | <0.005 | |
| Parity | 0 | 100 (41.0) | 63 (40.9) | 0.988 |
| ≥1 | 144 (59.0) | 91 (59.1) | ||
| Menopause | No | 212 (86.9) | 114 (74.0) | <0.005 |
| Yes | 32 (13.1) | 40 (26.0) | ||
| Marital status | No | 77 (31.6) | 58 (37.7) | 0.210 |
| Yes | 167 (68.4) | 96 (62.3) | ||
| Previous treatment. | No | 239 (98.0) | 150 (97.4) | 0.739 |
| Yes | 5 (2.0) | 4 (2.6) | ||
| Preoperative HPV loada) | + | 6 (2.5) | 2 (1.3) | <0.005 |
| ++ | 123 (50.4) | 49 (31.8) | ||
| +++ | 82 (33.6) | 77 (50.0) | ||
| Unknown | 33 (13.5) | 26 (16.9) | ||
| Multiplicity of HPV | Single | 177 (72.5) | 59 (38.3) | <0.001 |
| Multiple | 67 (27.5) | 95 (61.7) | ||
| Co-infection with low-risk HPV | No | 215 (88.1) | 108 (70.1) | <0.001 |
| Yes | 29 (11.9) | 46 (29.9) | ||
| Cytology | Normal | 6 (2.5) | 1 (0.7) | 0.232 |
| ASCUS | 62 (25.4) | 40 (26.1) | ||
| LSIL | 48 (19.7) | 44 (28.8) | ||
| ASC-H | 37 (15.2) | 17 (11.1) | ||
| HSIL | 87 (35.7) | 49 (32.0) | ||
| AGUS | 2 (0.8) | 0 (0.0) | ||
| SCC | 2 (0.8) | 2 (0.8) | ||
| CIN at treatment | CIN 2 | 71 (29.1) | 79 (51.3) | <0.001 |
| CIN 3 | 173 (70.9) | 75 (48.7) | ||
| Type of surgery | LEEP | 81 (33.3) | 62 (40.3) | 0.161 |
| Conization | 162 (66.7) | 92 (59.7) | ||
| Resection margin | Negative | 214 (88.1) | 122 (79.2) | <0.05 |
| Positive | 29 (11.9) | 32 (20.8) | ||
| Endocervical resection margin | Negative | 200 (94.3) | 136 (88.3) | <0.05 |
| Positive | 14 (5.7) | 18 (11.7) | ||
| Glandular involvement | No | 118 (48.4) | 82 (53.2) | 0.342 |
| Yes | 126 (51.6) | 72 (46.8) | ||
| Follow-up cytology | Normal/ASCUS/LSIL | 240 (98.4) | 133 (86.4) | <0.001 |
| ASC-H/HSIL | 4 (1.6) | 21 (13.6) | ||
| CIN persistence/recurrence | No | 239 (98.0) | 102 (66.2) | <0.001 |
| Yes | 5 (2.0) | 52 (33.8) | ||
| CIN 2+ persistence/recurrence | No | 244 (100.0) | 139 (90.3) | <0.001 |
| Yes | 0 (0.0) | 15 (9.7) | ||
Values are presented as mean±standard deviation or number (%).
hrHPV, high-risk human papillomavirus; ASCUS, atypical squamous cells of undetermined significance; LSIL, low grade squamous intraepithelial lesion; ASC-H, atypical squamous cells, cannot exclude HSIL; HSIL, high grade squamous intraepithelial lesion; AGUS, atypical glandular cells of undetermined significance; SCC, squamous cell carcinoma; CIN, cervical intraepithelial neoplasia; LEEP, loop electrosurgical excision procedure.
Table 2.
| Characteristics |
Univariate |
Multivariate |
|||||
|---|---|---|---|---|---|---|---|
| OR | 95% CI | P-value | OR | 95% CI | P-value | ||
| Age (yr) | ≥50 | 2.553 | 1.406–4.636 | <0.005 | 2.486 | 0.652–9.494 | 0.182 |
| <50 | |||||||
| Parity | ≥1 | 0.811 | 0.512–1.284 | 0.372 | |||
| 0 | |||||||
| Menopause | Yes | 2.577 | 1.355–4.899 | <0.010 | 1.085 | 0.257–4.586 | 0.912 |
| No | |||||||
| Marriage | Yes | 0.763 | 0.500–1.165 | 0.211 | |||
| No | |||||||
| Previous treatment | Yes | 1.151 | 0.303–4.374 | 0.836 | |||
| No | |||||||
| Preoperative HPV loada) | +++ | 2.231 | 1.329–3.746 | <0.010 | 2.063 | 1.139–3.737 | <0.050 |
| +-++ | |||||||
| Multiplicity of HPV | Multiple | 4.402 | 2.713–7.144 | <0.001 | 4.752 | 2.593–8.710 | <0.001 |
| Single | |||||||
| Co-infection with low risk HPV | Yes | 2.917 | 1.603–5.307 | <0.001 | 1.584 | 0.750–3.344 | 0.228 |
| No | |||||||
| Follow-up cytology | ASC-H/HSIL/SCC | 0.797 | 0.505–1.256 | 0.327 | |||
| Normal/ASCUS/LSIL | |||||||
| CIN at treatment | CIN 2 | 2.674 | 1.656–4.310 | <0.001 | 2.732 | 1.451–5.128 | <0.010 |
| CIN 3 | |||||||
| Type of surgery | Conization | 0.884 | 0.561–1.395 | 0.597 | |||
| LEEP | |||||||
| Resection margin | Positive | 1.936 | 1.117–3.353 | <0.05 | 1.319 | 0.558–3.121 | 0.528 |
| Negative | |||||||
| Glandular involvement | Yes | 0.762 | 0.484–1.201 | 0.242 | |||
| No | |||||||
OR, odd ratio; CI, confidence interval; HPV, human papillomavirus; ASC-H, atypical squamous cells, cannot exclude HSIL; HSIL, high grade squamous intraepithelial lesion; SCC, squamous cell carcinoma; ASCUS, atypical squamous cells of undetermined significance; LSIL, low grade squamous intraepithelial lesion; CIN, cervical intraepithelial neoplasia; LEEP, loop electrosurgical excision procedure.
Table 3.
Table 4.
| Variables | Values |
Multivariate |
|||
|---|---|---|---|---|---|
| OR | 95% CI | P-value | |||
| CIN after conization or LEEP (n=57) | |||||
| Menopause | Yes | 19/72 (26.4) | 3.969 | 1.733–9.088 | <0.001 |
| No | 38/326 (11.7) | ||||
| Preop HPV loada) | +++ | 30/159 (18.9) | 2.430 | 1.135–5.202 | <0.050 |
| +–++ | 18/178 (10.1) | ||||
| unknown | 9/61 (14.8) | ||||
| Postop HPV loada)b) | +++ | 18/35 (51.4) | 5.351 | 1.091–26.236 | <0.050 |
| +–++ | 27/100 (27.0) | ||||
| Negative | 12/244 (4.9) | ||||
| Multiplicity of HPV | Multiple | 31/162 (19.1) | 2.345 | 1.109–4.958 | <0.050 |
| Single | 26/236 (11.0) | ||||
| CIN 2/3 after conization or LEEP (n=15) | |||||
| Menopause | Yes | 6/72 (8.3) | 4.31 | 1.154–16.115 | <0.050 |
| No | 9/326 (2.8) | ||||
| Pathology before conization or LEEP | CIN 3 | 12/248 (4.8) | 10.87 | 2.146–55.046 | <0.010 |
| CIN 2 | 3/150 (2.0) | ||||
| Postop HPV loada)b) | +++ | 7/35 (20.0) | 4.24 | 1.201–14.963 | <0.050 |
| +–++ | 7/100 (7.0) | ||||
Table 5.
| No. | Age (yr) | Parity | Menopause | HPV loada) | HPV Type | Treatment | Cytology | Pathology | RM | GI |
Follow up |
Time to diagnosis of residual/recurrent CIN 2/3 (mon) | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HPV | Type change | Cytology | Path | ||||||||||||
| 1 | 25 | 0 | No | +++ | 161,833 | Cone | ASCUS | CIN 2 | - | - | 161,833 | - | HSIL | CIN2 | 11.7 |
| 2 | 27 | 0 | No | +++ | 3,339 | LEEP | ASCUS | CIN 3 | + | - | 16 | + | LSIL | CIN3 | 8.4 |
| 3 | 27 | 0 | No | ++ | 52 | Cone | HSIL | CIN 3 | + | + | 52 | - | ASCUS | CIN3 | 9.3 |
| 4 | 38 | 1 | No | ++ | 1,618 | LEEP | ASCUS | CIN 3 | - | + | 183,553 | - | ASCUS | CIN2 | 23.1 |
| 5 | 40 | 2 | No | ++ | 33 | Cone | HSIL | CIN 3 | + | + | 33 | - | HSIL | CIN3 | 13.3 |
| 6 | 41 | 2 | No | ++ | 33 | Cone | HSIL | CIN 3 | - | + | 3,556 | + | ASCUS | CIN2 | 12.5 |
| 7 | 42 | 1 | No | +++ | 31 | Cone | LSIL | CIN 3 | + | + | 3,159 | - | HSIL | CIN3 | 7.7 |
| 8 | 45 | 4 | No | +++ | 16 | Cone | HSIL | CIN 3 | - | + | 16 | - | HSIL | CIN3 | 9.5 |
| 9 | 49 | 1 | No | ++ | 5,868 | Cone | HSIL | CIN 3 | - | - | 5,868 | - | HSIL | CIN3 | 2.1 |
| 10 | 52 | 2 | Yes | ++ | 31 | Cone | HSIL | CIN 3 | + | + | 31 | - | ASCUS | CIN3 | 13.3 |
| 11 | 56 | 2 | Yes | ++ | 5,358 | LEEP | HSIL | CIN 2 | - | - | 5,358 | - | LSIL | CIN2 | 4.4 |
| 12 | 56 | 2 | Yes | +++ | 16 | Cone | HSIL | CIN 2 | - | - | 16 | - | ASCUS | CIN3 | 6.7 |
| 13 | 57 | 2 | Yes | ++ | 1,635 | LEEP | SCC | CIN 3 | - | - | 1,635 | - | HSIL | CIN2 | 4.3 |
| 14 | 59 | 2 | Yes | ++ | 3,352 | LEEP | HSIL | CIN 3 | - | - | 3,352 | - | HSIL | CIN3 | 5.7 |
| 15 | 63 | 3 | Yes | + | 45 | LEEP | HSIL | CIN 3 | - | + | 45 | - | HSIL | CIN3 | 4.7 |
HPV, human papillomavirus; RM, resection margin; GI, glandular involvement; Path, pathology; Cone, conization; ASCUS, atypical squamous cells of undetermined significance; HSIL, high-grade intraepithelial lesion; LEEP, loop electrosurgical excision procedure; LSIL, low-grade intraepithelial lesion; SCC, squamous cell carcinoma.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download