Introduction
Pelvic organ prolapse (POP) is a descent of one or more aspects of the vagina and uterus, presumably due to defects in the pelvic organ support system. POP is a common condition in women, with prevalence rates ranging from 41% to 50% [
1]. The lifetime risk of undergoing a single operation for POP or urinary incontinence is approximately 11% [
2].
Many studies have been conducted in order to determine the risk factors for POP. Increasing parity and aging are established risk factors for POP; however, these factors are not modifiable [
3,
4]. Obesity is a modifiable risk factor; however, studies on the relationship between obesity and POP have shown inconsistent results. Many of these studies did not use a validated tool for the evaluation of POP. Obesity is associated with pelvic floor symptoms, as indicated by previous studies [
5,
6].
Understanding the impact of obesity on POP is crucial as obesity is a modifiable risk factor whose prevalence is increasing, especially in women [
7]. The diagnostic criteria for obesity differs between Western and Asian women. Moreover, studies in Asian women are scarce. The objective of this study was to evaluate the correlation between obesity and POP, both anatomically and symptomatically, in Korean women.
Go to :

Materials and methods
We retrospectively reviewed the medical records of patients whose chief complaints were related to POP, and who visited the CHA Bundang Medical Center from January 2013 to December 2016. All of the enrolled women were Korean.
Each patient underwent a comprehensive physical examination, which included a standardized Pelvic Organ Prolapse Quantification (POP-Q) examination for the anatomical assessment of POP. A POP-Q examination was performed by a practitioner with the patient in the lithotomy position during the valsalva strain. At the initial visit, we recorded height, weight, and results of the POP-Q assessment: Ba as the most distal point of the upper anterior vaginal wall, Bp as the most distal point of the posterior vaginal wall, C as the most distal edge of the cervix or vaginal cuff, and total vaginal length (TVL) as the total length of the vagina. The POP degree was represented by the POP stage from 0 to IV based on the POPQ system [
8]. Each patient was also asked to complete the PFDI-20 questionnaire at the initial visit. The PFDI-20 is a validated questionnaire used to assess the degree of pelvic floor symptom distress and is composed of 3 subscales: Pelvic Organ Prolapse Distress Inventory (POPDI)-6, Colorectal Anal Distress Inventory (CRADI)-8, and Urinary Distress Inventory (UDI)-6. Here, we used the PFDI-20 Korean translated version [
9].
We evaluated obesity based on the patient’s BMI. Generally, obesity is defined as a BMI >30 kg/m
2 and overweight is defined as a BMI 25–30 kg/m
2 according to the World Health Organization (WHO) guidelines [
10]. However, here, we defined obesity as BMI >25 kg/m
2 as defined previously for the Asia-Pacific population [
10].
We analyzed the correlation between obesity and POP using continuous variables and dichotomous variables. We then separately evaluated the correlation between obesity and POP for patients with advanced POP (stages III and IV). We excluded women with incomplete records of height and weight, or POP stage <I at the initial visit. We also excluded women who were pregnant and/or underwent prior surgery due to POP.
We used 2 statistical methods. Pearson’s correlation coefficient was used to analyze the correlation of BMI as a continuous variable with POP-Q points and PFDI-20 scores. An independent samples t-test was used to evaluate differences between the groups (obese/non-obese) for obesity as a dichotomous variable. The results were considered significant if the P<0.05. All statistical analysis was performed using SPSS software (version 24; SPSS Inc., Chicago, IL, USA).
Go to :

Results
In total, 533 women were reviewed and the following women were excluded: 27 patients with incomplete records of height and weight, 3 with POP stage ≤I, 15 who underwent hysterectomy due to POP, 8 who underwent prior prolapse surgery, and 4 who were pregnant at the initial visit. Ultimately, 476 women who met the inclusion criteria were enrolled and completed the POP-Q exam. Among the 476 enrolled patients, 370 (77.7%) patients completed the PFDI20 questionnaire, 223 (80.9%) patients were in the nonobese group, and 147 (78.2%) patients were in the obese group. The study subjects had a mean age of 65.56±11.27 years (91.6% were postmenopausal), a mean BMI of 24.35±3.08 kg/m
2, and a mean parity of 3.19±1.50 (
Table 1). The largest group from the POP-Q belonged to stage III and the distribution of the POP-Q points are described in
Table 2. All subjects were divided into two groups, an obese group (n=188, 39.5%) and a non-obese group (n=288, 60.5%), based on the BMI cut-off value of 25 kg/m
2. There were no statistically significant differences in age, parity, and/or prior hysterectomy between the two groups.
Table 1.
Demographics and clinical characteristics of patients (n=476)
|
Variables |
No. (%) |
Mean±SD |
|
Mean age (yr) |
|
65.56±11.27 |
|
Mean BMI (kg/m²) |
|
24.35±3.08 |
|
<25 (non-obese) |
288 (60.5) |
|
|
≥25 (obese) |
188 (39.5) |
|
|
≥25 and <30 |
167 (88.8) |
|
|
≥30 |
21 (11.1) |
|
|
Mean parity |
|
3.19±1.50 |
|
Post menopausal status |
436 (91.6) |
|
|
Prior pessary use |
23 (4.8) |
|
|
Prior hysterectomy |
43 (9.3) |
|
|
Hormonal therapy |
4 (0.8) |
|
|
POP-Q stage |
|
|
|
Stage II |
139 (29.2) |
|
|
Stage III |
293 (61.6) |
|
|
Stage IV |
44 (9.2) |
|
|
Race |
|
|
|
Asian |
476 (100.0) |
|

Table 2.
Distribution of pelvic organ prolapse (POP) stage (n=476)
|
POP-Q points |
Stage 0 |
Stage I |
Stage II |
Stage III |
Stage IV |
|
Ba |
5 (1.0) |
49 (10.3) |
145 (30.5) |
240 (50.4) |
37 (7.8) |
|
Bp |
11 (2.3) |
100 (21.0) |
259 (54.4) |
69 (14.5) |
37 (7.8) |
|
C |
17 (3.6) |
188 (39.5) |
89 (18.7) |
141 (29.6) |
41 (8.6) |

To evaluate the correlation between obesity and POP anatomically, we calculated Pearson`s correlation coefficient for BMI and POP-Q points (Ba, Bp, and C). There were no statistically significant correlations between BMI or each POPQ point (
Table 3,
Fig. 1). In the same manner, to evaluate the correlation with obesity and POP symptomatically, we calculated Pearson’s correlation coefficient for BMI and PFDI-20 and its subscales (POPDI-6, CRADI-8, and UDI-6). There was no statistically significant correlations between BMI and PFDI20 (
Table 3,
Fig. 2). In the same manner, we also evaluated the correlation between obesity and POP for patients with severe POP (stage>III). There was no statistically significant correlations between BMI, POP-Q, or PFDI-20 (
Table 4) in patients with severe POP. All enrolled patients were dichotomously classified as obese or non-obese. We then separately analyzed the difference between the anatomic and symptomatic correlations between the two groups. No statistically significant anatomical or symptomatic difference was found between the obese and the non-obese groups (
Table 5).
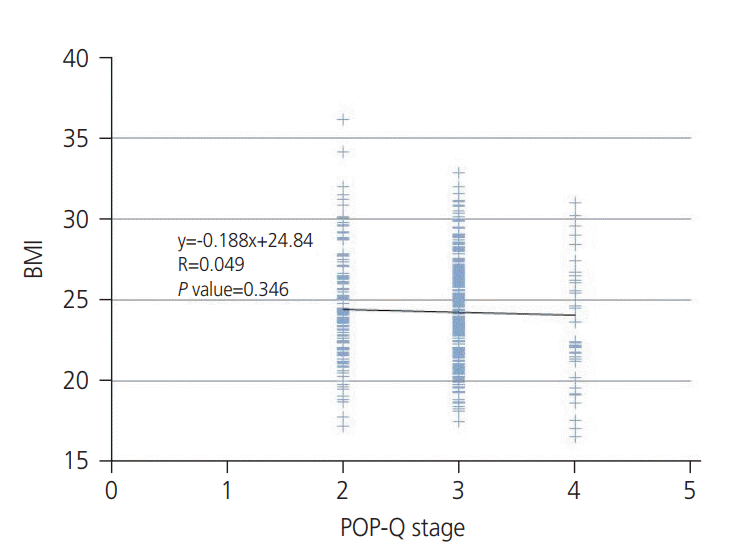 | Fig. 1.Correlation between body mass index (BMI) and Pelvic Organ Prolapse Quantification (POP-Q) stage. BMI was not significantly correlated with POP-Q stage (R=0.049, P=0.346). 
|
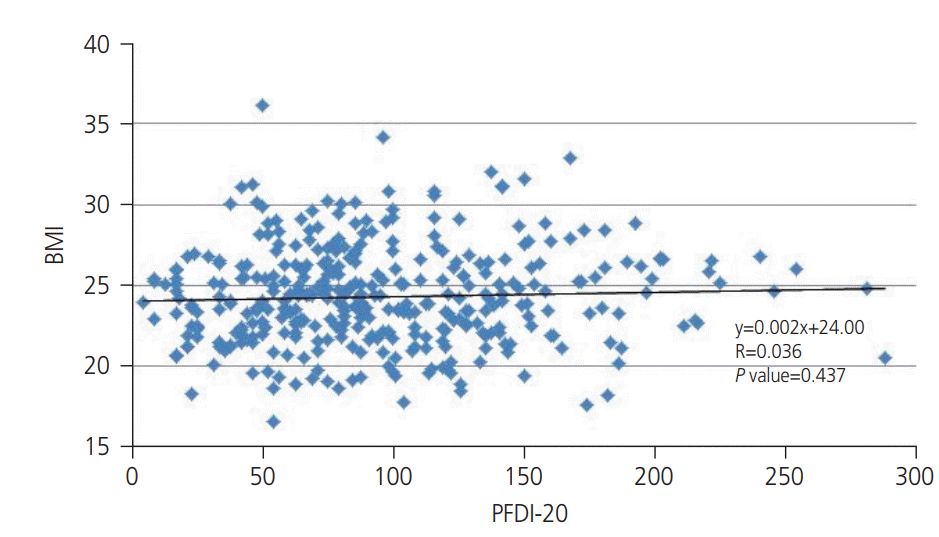 | Fig. 2.Correlation between body mass index (BMI) and Pelvic Floor Distress Inventory (PFDI)-20. BMI was not significantly correlated with PFDI-20 (R=0.036, P=0.437). 
|
Table 3.
Correlation between body mass index (BMI) with Pelvic Organ Prolapse Quantification (POP-Q) points and Pelvic Floor Distress Inventory (PFDI)-20
|
Variables |
Pearson’s correlation coefficient |
P-value |
|
POP-Q points |
|
|
|
Ba |
0.022 |
0.633 |
|
Bp |
−0.042 |
0.363 |
|
C |
−0.050 |
0.277 |
|
PFDI-20 |
|
|
|
POPDI-6 |
0.056 |
0.286 |
|
CRADI-8 |
0.003 |
0.960 |
|
UDI-6 |
0.051 |
0.340 |
|
PFDI-20 |
0.048 |
0.355 |

Table 4.
Correlation between body mass index (BMI) with Pelvic Organ Prolapse Quantification (POP-Q) points and Pelvic Floor Distress Inventory (PFDI)-20 for ≥stage 3 (n=337)
|
Variables |
Pearson’s correlation coefficient |
P-value |
|
POP-Q point |
|
|
|
Ba |
0.026 |
0.571 |
|
Bp |
−0.040 |
0.381 |
|
C |
−0.055 |
0.227 |
|
PFDI-20 |
|
|
|
POPDI-6 |
0.048 |
0.359 |
|
CRADI-8 |
0.005 |
0.924 |
|
UDI-6 |
0.049 |
0.357 |
|
PFDI-20 |
0.045 |
0.391 |

Table 5.
Difference in Pelvic Organ Prolapse Quantification (POPQ) points and Pelvic Floor Distress Inventory (PFDI)-20 between obese/non-obese groups
|
Variables |
Obese group |
Non-obese group |
P-value |
|
POP-Q points |
|
|
|
|
Ba |
1.9±2.3 |
1.5±2.3 |
0.052 |
|
Bp |
0.3±2.2 |
0.3±2.5 |
0.874 |
|
C |
−0.1±3.8 |
−0.3±3.6 |
0.574 |
|
PFDI-20 |
|
|
|
|
POPDI-6 |
47.5±23.9 |
43.2±22.4 |
0.083 |
|
CRADI-8 |
19.9±18.8 |
19.0±18.2 |
0.537 |
|
UDI-6 |
33.6±24.1 |
30.3±22.0 |
0.191 |
|
PFDI-20 |
99.1±53.7 |
90.8±49.7 |
0.131 |

Go to :

Discussion
Obesity has been consistently identified as a risk factor for pelvic floor disorder. The most probable mechanism of POP development in obese women is the increased intraabdominal pressure that causes weakening of the pelvic floor muscles and fascia [
11]. However, previous studies that evaluated the relationship between obesity and POP illustrated inconsistent results. The prevalence of obesity has been increasing globally, and a better understanding of how obesity influences POP is crucial as unlike age and parity, obesity is a modifiable factor.
In our study, obesity was not shown to be significantly related to POP anatomically or symptomatically. In contrast to our study, Hendrix et al. reported that there was a significant association between BMI and POP in overweight (BMI 25–30 kg/m
2) and obese women (BMI >30 kg/m
2) via data from the Women’s Health Initiative study [
12]. In that study, POP was assessed by visual inspection during the valsalva maneuver in the supine position, not by a standardized POPQ exam. Shalom et al. [
13] evaluated the correlation between obesity and POP by BMI and a standardized POP-Q exam. Consistent with our study, they reported that no significant correlation was established between BMI and POP severity. Giri et al. published a systematic review and meta-analysis of the association between obesity and POP [
14], and dichotomously assessed the risk ratio between obese and nonobese women (as defined by WHO). In contrast to our study, obesity was significantly associated with POP in their study. Compared with normal weight women (BMI <25 kg/m
2), overweight women (BMI 25–30 kg/m
2) and obese women (BMI >30 kg/m
2) had risk ratios of at least 1.36 (95% confidence interval [CI], 1.20–1.53) and 1.47 (95% CI, 1.35–1.59), respectively. The authors did not include studies that used a continuous variable analysis. Vergeldt et al. [
15] performed a systematic review of risk factors for POP and recurrence. In that study, a higher BMI as a dichotomous variable was a significant risk factor; however, BMI as a continuous variable had no association with POP. We performed dichotomous variable and continuous variable analyses to confirm the discrepancy between the two analyses. However, there were no significant correlations between either analyses.
Symptom distress due to POP is clinically significant and an investigation of the correlation between obesity and POP symptoms via a validated tool is also important. However, the correlation between obesity and POP symptom distress has been inconsistently reported in previous studies. We evaluated the correlation by a validated tool (PFDI-20 and its subscales), and no significant correlation was noted in our study. Uustal Fornell et al. [
16] reported that being overweight or obese were strongly related to urinary and fecal incontinence, but not to POP symptoms. Bradley et al. [
17] did not find a positive correlation between obesity and prolapse symptoms. This is consistent with our results. We also attempted to evaluate the correlation in patients with more severe prolapse. However, there was no significant correlation between obesity and POP anatomically or symptomatically in patients with severe POP (greater than stage III). Consistent with our findings, Shalom et al. [
13] and Washington et al. [
18] found that there was no significant correlation between BMI and prolapse severity.
With respect to obesity, there are differences between Korean women and Western women. According to the WHO guidelines, the cut-off value of obesity is defined as a BMI >30 kg/m
2, which was the cut-off value used in previous studies. However, the WHO obesity cut-off value for obesity for Asians is 25 kg/m
2; therefore, here, we defined obesity as a BMI >25 kg/m
2. In our study, only 4.4% (21/476) of the patients had a BMI >30 kg/m
2. In contrast, 33.4% of US women had a BMI >30 kg/m
2 [
19]. Therefore, with respect to the correlation between obesity and POP, a study for Korean women may have clinical significance.
However, studies for Korean women are rare and have demonstrated conflicting results. Kim et al. evaluated the risk factors for pelvic organ prolapse, and found that BMI was not significantly correlated with POP stage (
P=0.271) [
20]. Seo and Kim [
21] evaluated pelvic organ support and the prevalence of POP via the POP-Q exam for Korean women. In their study, BMI showed a significant correlation with increasing POP-Q stage (
P<0.001). Furthermore, the correlation of obesity and POP was not the primary aim in the above studies. To our knowledge, this is the first study that comprehensively evaluated the relationship between obesity and POP in Korean women. Although controversy exists, most of the studies for Western women showed a significant correlation between obesity and POP. Though diagnostic criteria for obesity is different between Western and Asian women, the present study showed that there was no significant correlation between obesity and POP severity anatomically or symptomatically in Korean women. The cause of different results between Western and Asian women is not clear. Further large, well-designed studies in Asian women may explain the discrepancy in these results.
The strength of this study is that we evaluated the correlation between obesity and POP anatomically as well as symptomatically. Furthermore, we also analyzed the data by dichotomous and continuous variables. Another strength is that we used a standardized tool for the anatomic and symptomatic evaluation of POP (POP-Q was used for anatomic evaluation, and PFDI-20 for symptomatic evaluation). There are some limitations of this study. First, this was a retrospective and cross-sectional study. Second, the enrolled women were restricted to patients with POP, therefore potentially limiting the generalizability of our results. Third, all patients underwent the POP-Q exam, but not all patients completed the PFDI-20, which may result in bias in the interpretation.
In conclusion, obesity was not significantly correlated with POP severity, anatomically or symptomatically, in Korean women. As noted earlier, diagnostic criteria for obesity is different between Western and Asian women and studies for Asian women are very scarce. Thus, further studies in Asian women are essential in order to confirm our findings.
Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download