This article has been
cited by other articles in ScienceCentral.
Abstract
The intrauterine device (IUD) is the most common method of reversible contraception in women. However, IUD can perforate the uterus and also migrate into pelvic or abdominal organs. A 43-year-old woman with a 5-year history of IUD placement and without specific symptoms, decided to remove her IUD and undergo tubal ligation. Radiological assessment, including a pelvic X-ray and ultrasonography, revealed no copper IUD within the uterus. Retrieval attempts with cystoscopy were unsuccessful. The IUD was found embedded in the fundal part of the bladder wall and was subsequently removed through a laparotomy incision. Although there are cases in the literature that were successfully managed with cystoscopy, in chronic cases, the formation of granulation tissue may preclude retrieval of an IUD using this intervention.
Go to :

Keywords: Intrauterine devices, Contraception, Migration, Bladder
Introduction
Intrauterine devices (IUDs) are effective, safe, and widely used birth control methods, accounting for 16.5% of birth control used in undeveloped countries and 9.4% of birth control used in developed countries [
1]. The incidence of uterine perforation by IUD is reported to be between 1.3 and 1.6 per 1,000 insertions [
2], indicating perforation is a relatively infrequent but potentially serious complication. Perforations may occur either immediately, by improper insertion, or years after insertion by device migration. We report a case of an IUD that penetrated the bladder wall and became symptomatic 5 years after insertion.
The IUD is used by more than 150 million women around the world, making it the most widely used reversible method of contraception [
1]. Although IUDs are commonly considered to be safe, it also has some serious complications. Uterine perforation due to an IUD is seen in 0.05 to 13 cases out of 1,000 IUD placements [
2]. Following the uterine rupture, an IUD may potentially migrate to the pelvic or intra-abdominal cavity, causing several complications. A literature review of the 18 years until 1999 showed 165 reported cases of migrated IUD, which shows that migration to the bladder is uncommon and has been reported in only 31 cases [
3]. The United Kingdom Selected Practice Recommendations recommends a follow-up visit after the first menses, or 3–6 weeks after insertion, to exclude infection, perforation, or expulsion [
4].
Go to :

Case report
A 43-year-old woman—gravid 7, live 7—was referred to the Jahrom University of Medical Science Gynecologic Clinic with complaints of unspecified lower abdominal pain and dysuria. These symptoms had persisted for three months, despite repeated treatments for urinary tract infections by several gynecologists. She had a history of a copper-T IUD insertion 5 years prior to presentation. The patient’s documents were reviewed, and included tubal ligation consent, pelvic ultrasound, and pelvic X-ray. On pelvic X-ray, her IUD appeared to be upside down (in the reverse position). Further, in a report of an ultrasound performed two months before surgery, the radiologist noted that the IUD was unable to be visualized in the endometrial cavity. There was a linear echogenic structure in the myometrium of the anterior fundal part of the bladder, measuring about 22×2 mm, which might have been a migrated IUD. A T-shape IUD was inserted by a midwife in the patient’s village 5 years ago, but the patient did not present for routine follow-ups after insertion prior to her decision to pursue tubal ligation. During the years following insertion, she was asymptomatic; however, for the past 2 to 3 months she experienced pyuria and leukocytes in her urine, though all urine cultures were normal during this period.
During an abdominal-pelvic examination, mild tenderness was found in the suprapubic area during deep palpation. The IUD threads were visible during inspection of the vaginal canal, so the gynecologist tried to remove the IUD but was unsuccessful. The patient opted to have the IUD removed in the operating room, followed by tubal ligation. Preoperative laboratory findings were normal with the exception of urinalysis, which showed pyuria and leukocytes. Her urine culture was normal, so she started broad-spectrum antibiotics and was transferred to the operating room. During laparotomy, the gynecologist observed severe adhesions between the large intestine, the posterior part of the fundal uterus, and bladder. Tubal ligation was done despite difficulty visualizing both tubes due to adhesions and the abdominal wall was closed. Then, the gynecologist tried to remove the IUD through the vaginal canal, but was unsuccessful. The gynecologist then consulted with an expert professor of gynecology who tried to remove the IUD through the vaginal canal but was unsuccessful. The bulging point of the left anterior vesical wall was visible and was ultimately determined to be the ramus of the IUD following uterine perforation. An immediate consultation was made with a urologist. The IUD and the stones caused by the IUD were visualized by cystoscopy; however, these were not able to be removed through cystoscopy.
The IUD and stones were ultimately removed by the urologist through an incision on the anterior vesical wall. After IUD removal and bladder repair, a posterior uterine wall perforation was repaired. This perforation was 1×2 cm in size and was likely caused by the failed attempts to remove the IUD through the vaginal canal.
The patient was discharged on hospital day seven and experienced an uneventful postoperative period (
Fig. 1).
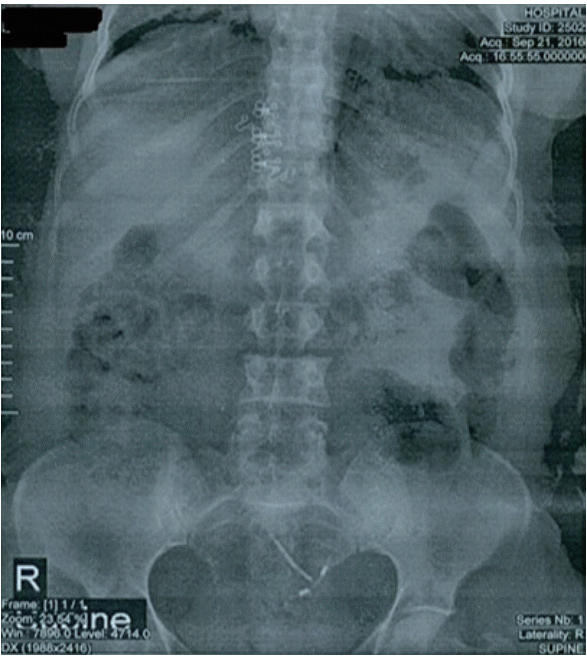 | Fig. 1.Migrated intrauterine device and stone seen on abdominal plain X-ray. In abdominal plain X-ray, intrauterine device (IUD) looks upside down (in reverse position) in uterine cavity, it’s also left leaning instead of longitudinal position along the middle line of the uterus .A few stones has been formed on the right branch of the IUD which shows passage of the time. 
|
A plain frontal supine abdominal X-ray showed a T-shaped IUD on the left side of the pelvis with a radio-opaque stone (
Fig. 2).
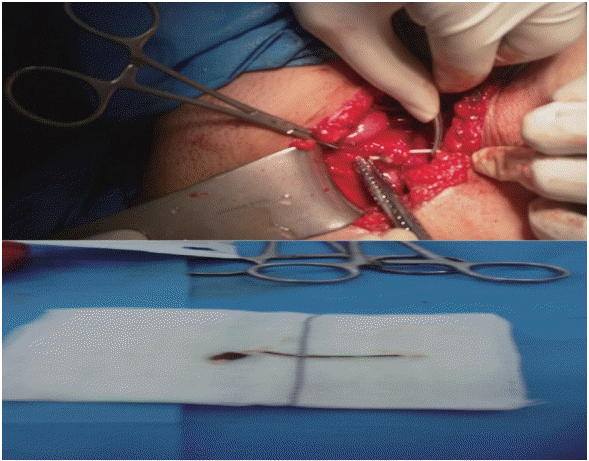 | Fig. 2.Removal of the intrauterine device (IUD) during cystostomy. During laparotomy, left branch of the IUD was seen as a bulging point in the left side of fundus of bladder, so through a transverse incision on the bladder and despite the severe adhesion, the IUD was removed and the incision site was repaired. 
|
Vaginal ultrasound showed a linear echogenic structure in the myometrium of the anterior fundal area of the bladder, measuring about 22×2 mm, which may have been the migrated IUD.
Go to :

Discussion
The IUD is a popular contraceptive method. Although IUDs are commonly considered safe, they are occasionally associated with serious complications such as pelvic pain, bleeding, spotting, increased risk of pelvic inflammatory disease, and unexpected pregnancies [
3]. Uterine perforation is an uncommon IUD complication. Known risk factors for uterine perforation include inadequate training of family planning providers, insertion during the early puerperal period when the uterus is soft and bulky, past history of perforation, and an anatomically highly flexed uterus [
5]. The overall reported incidence of IUD perforation is about 0.87 per 1,000 insertions [
6]. IUD migration into the peritoneal cavity and uterine structures is another rare complication of this contraception method.
Because of the rarity of bladder perforation by an IUD, it may be misdiagnosed. While some patients experience hematuria, lower abdominal pain, and irritative urinary symptoms, others may experienced a mild complications. Complete migration of an IUD into the bladder cavity can also lead to stone formation. To date, half of the cases with IUD migration to the bladder presented with stones that varied in size from 1–10 cm. Foreign bodies in the bladder cavity may act as a nidus for stone formation, and infections may also serve as predisposing factors. The presence of urinary tract symptoms and a history of IUD insertion with failure to locate the threads may indicate device migration [
7]. The present case presented with repeated episodes of cystitis, which were cured following administration of antispasmodic and antibiotic therapies.
To determine the location of the migrated IUD, different imaging modalities have been used. The transvaginal and transabdominal-ultrasonography approaches are useful methods for detecting IUD migration [
8]. Abdominal X-ray is the preliminary modality for investigating IUD migration, especially for the detection of stones caused by IUDs. In some cases, computed tomography is needed for diagnosis [
7]. Cystoscopy is another means of visualizing the intravesical IUD and may assist with removal [
9]. The accepted treatment for IUD-associated perforations is abdominal surgery. Initially, this was accomplished via laparotomy; however, as surgical techniques have developed, laparoscopy is often used [
10].
It is not known when the IUD migrated to the bladder: during insertion, during intercourse, due to hard work, or because of unknown causes. So, for earlier detection of IUD migration and preventing its complications, regular follow-ups are highly recommended. The cause of IUD migration in this patient was unknown and she has not returned for follow-up.
For women with IUDs who have bladder stones and recurrent urinary tract infections, migration of the IUD into the bladder must be considered as a differential diagnosis. Furthermore, a simple abdominal X-ray and—if needed—cystoscopy can be very useful imaging modalities for patients who complain of unexplained urinary symptoms or pelvic pain.
We would like to address some important points about IUD migration which were ignored during the treatment of our case. Even when the IUD threads are visible in the vagina, removal may not be easy. Consequently, radiologic images are essential. This is particularly true when the IUD is not visible in the uterine cavity on ultrasonography reports. Another important point is that attempts to remove an IUD during laparotomy should occur prior to closing the abdomen wall to prevent another laparotomy.
Go to :

ACKNOWLEDGMENTS
The authors wish to thank Dr. Inaloo (urologist), Dr. Taheri (general surgeon), Professor Samad Farzinnia (editor), and the operating room staff of Jahrom Mohahari Hospital, Iran.
We would also like to thank the Clinical Research Development Unit of Paymanieh Educational and the Research and Therapeutic Center of Jahrom University of Medical Sciences for providing facilities for this work. We thank Professor Samad Farzinnia for his excellent assistance with this article.
Go to :

Notes
Go to :

References
1. Kaneshiro B, Aeby T. Long-term safety, efficacy, and patient acceptability of the intrauterine Copper T-380A contraceptive device. Int J Womens Health. 2010; 2:211–20.
2. Istanbulluoglu MO, Ozcimen EE, Ozturk B, Uckuyu A, Cicek T, Gonen M. Bladder perforation related to intrauterine device. J Chin Med Assoc. 2008; 71:207–9.

3. Aghaways I, Anwer Wahid S, Ali RH, Sabir F, Kakamad FH. Migration of an intrauterine device to the left inguinal region, the first reported case. Int J Surg Case Rep. 2016; 28:68–70.

4. Faculty of Family Planning and Reproductive Health Care; Royal College of Obstetricians and Gynecologists. UK selected practice recommendations for contraceptive use. London: FFPRHC and RCOG;2003.
5. Akpinar F, Ozgur EN, Yilmaz S, Ustaoglu O. Sigmoid colon migration of an intrauterine device. Case Rep Obstet Gynecol. 2014; 2014:207659.

6. Shin DG, Kim TN, Lee W. Intrauterine device embedded into the bladder wall with stone formation: laparoscopic removal is a minimally invasive alternative to open surgery. Int Urogynecol J Pelvic Floor Dysfunct. 2012; 23:1129–31.

7. Tosun M, Celik H, Yavuz E, Çetinkaya MB. Intravesical migration of an intrauterine device detected in a pregnant woman. Can Urol Assoc J. 2010; 4:E141–3.

8. Sepúlveda WH, Ciuffardi I, Olivari A, Gallegos O. Sonographic diagnosis of bladder perforation by an intrauterine device. A case report. J Reprod Med. 1993; 38:911–3.
9. Gyasi-Sarpong CK, Maison PO, Morhe E, Aboah K, Appiah KA, Azorliade R, et al. Intravesical migration of an intrauterine device. BMC Res Notes. 2016; 9:4.

10. Kaislasuo J, Suhonen S, Gissler M, Lähteenmäki P, Heikinheimo O. Uterine perforation caused by intrauterine devices: clinical course and treatment. Hum Reprod. 2013; 28:1546–51.

Go to :







 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download