INTRODUCTION
MATERIAL AND METHODS
Experimental design and animals
TC, TG, and BA levels in plasma, liver, and feces
Liver histology
Western blot analysis
Statistical analysis
RESULTS
Effects of seaweed supplementation on regulation of blood glucose levels in C57BL/6N mice fed a HFD
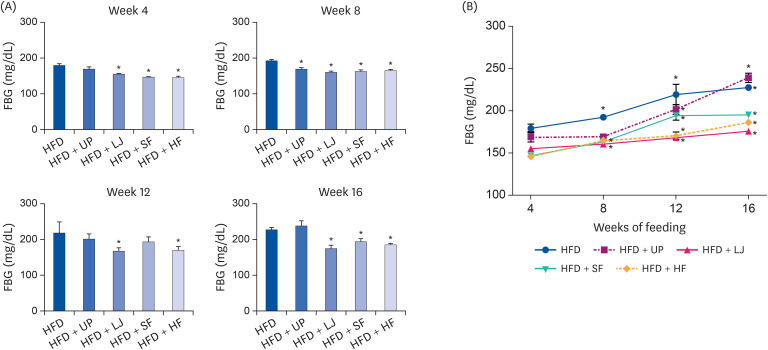 | Fig. 1Alteration of plasma FBG levels during the 16-week period of HFD or HFD supplementation with 4 types of seaweed in C57BL/6N mice. (A) Plasma FBG levels at weeks 4, 8, 12, and 16 of the experimental diet present significant differences between the seaweed supplemented groups compared to the HFD group every 4 weeks. (B) Alteration of plasma FBG levels measured every 4 weeks during the entire 16 weeks of feeding in C57BL/6N mice. Values are the mean ± standard error of the mean (n = 6 mice per group). An asterisk indicates a significant difference compared to either the HFD group (P < 0.05) or week 4 values of each group in (A) and (B), respectively.FBG, fasting blood glucose; HFD, high fat diet; UP, Undaria pinnatifida; LJ, Laminaria japonica; SF, Sargassum fulvellum; HF, Hizikia fusiforme.
|
Effects of seaweed supplementation on TC and TG levels in the liver and plasma of DIO mice
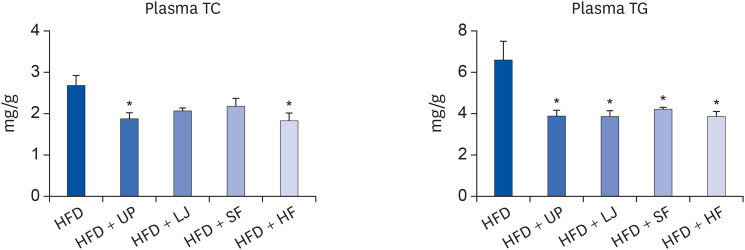 | Fig. 2Effects of seaweed supplementation on plasma TC and TG levels of C57BL/6N mice fed a HFD or seaweed supplemented HFD for 16 weeks. Values are the mean ± standard error of the mean (n = 6 mice per group). An asterisk indicates a significant difference compared to the HFD group (P < 0.05).TC, total cholesterol; TG, triglyceride; HFD, high fat diet; UP, Undaria pinnatifida; LJ, Laminaria japonica; SF, Sargassum fulvellum; HF, Hizikia fusiforme.
|
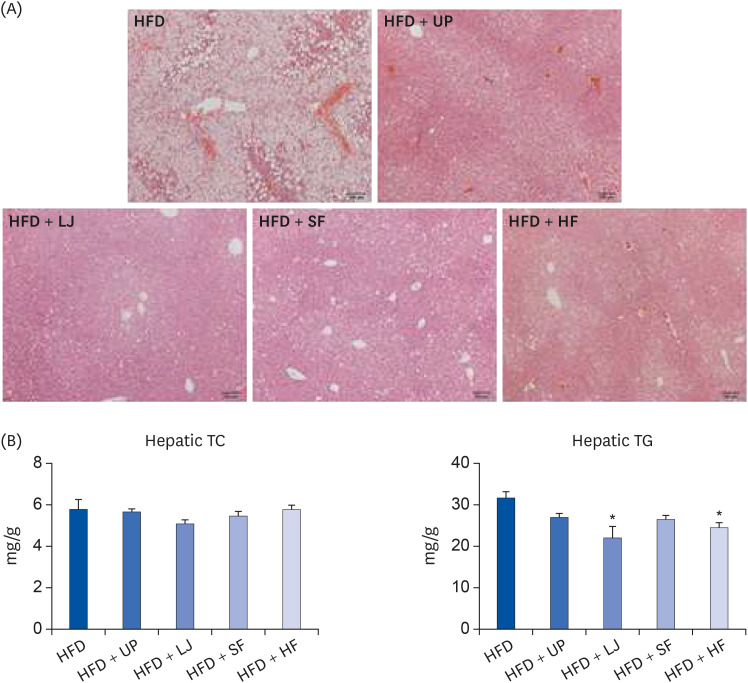 | Fig. 3Protective effects of seaweed supplementation on hepatic lipid accumulation in diet-induced obese mice. (A) Representative hematoxylin and eosin staining of livers from mice fed a HFD or seaweed supplemented HFD for 16 weeks, (B) Liver TG and TG contents of mice fed a HFD or seaweed supplemented HFD for 16 weeks (n = 5–6). Values are the mean ± standard error of the mean. An asterisk indicates a significant difference compared to the HFD group (P < 0.05).HFD, high fat diet; UP, Undaria pinnatifida; LJ, Laminaria japonica; SF, Sargassum fulvellum; HF, Hizikia fusiforme; TC, total cholesterol; TG, triglyceride.
|
Alterations of TC, TG, and BA excretion in feces of DIO mice by seaweed supplementation
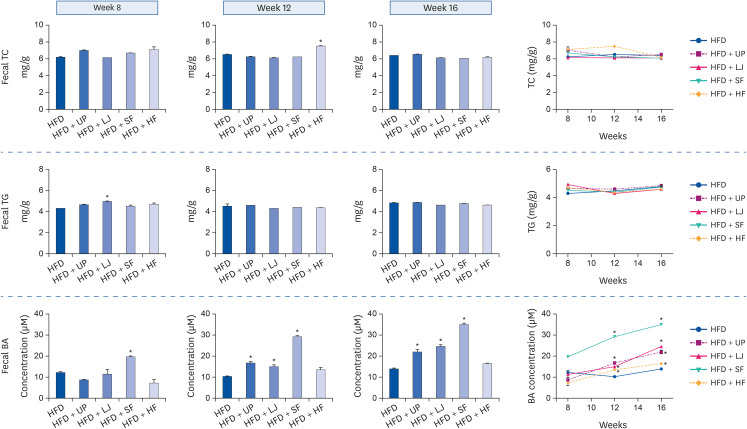 | Fig. 4Alterations of fecal TC, TG, and BA contents by seaweed supplementation during the latter half of the HFD feeding period in C57BL/6N mice. Feces samples from individual mouse cages were collected in order to measure fecal TC, TG, and BA levels as described in the Methods. Values are the mean ± standard error of the mean (n = 4–5). An asterisk in the bar graphs indicates a significant difference compared to HFD feeding only, whereas an asterisk in the connecting line graphs indicates a significant difference compared to week 8 (P < 0.05).HFD, high fat diet; UP, Undaria pinnatifida; LJ, Laminaria japonica; SF, Sargassum fulvellum; HF, Hizikia fusiforme; TC, total cholesterol; TG, triglyceride; BA, bile acid.
|
Effects of seaweed supplementation on regulation of insulin signaling and lipogenesis-related proteins in DIO mice
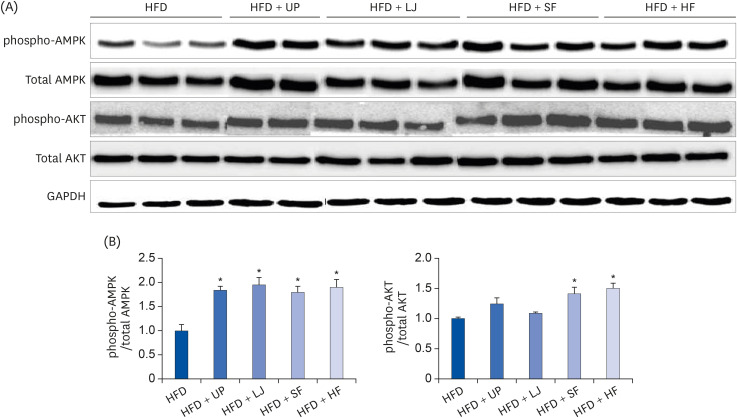 | Fig. 5Effects of insulin signaling-related proteins via seaweed supplementation in the livers of diet-induced obese mice. (A) Representative immunoblot analysis of phospho-AMPK and total AMPK, phospho-AKT, total AKT, and GAPDH levels in the liver, (B) Intensity of phospho-AMPK was normalized to total AMPK, and intensity of phospho-AKT was normalized to total AKT. Values are the mean ± standard error of the mean. An asterisk indicates a significant difference compared to the HFD group (P < 0.05).UP, Undaria pinnatifida; LJ, Laminaria japonica; SF, Sargassum fulvellum; HF, Hizikia fusiforme; AMPK, 5′-AMP-activated protein kinase; AKT, protein kinase B; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HFD, high fat diet.
|
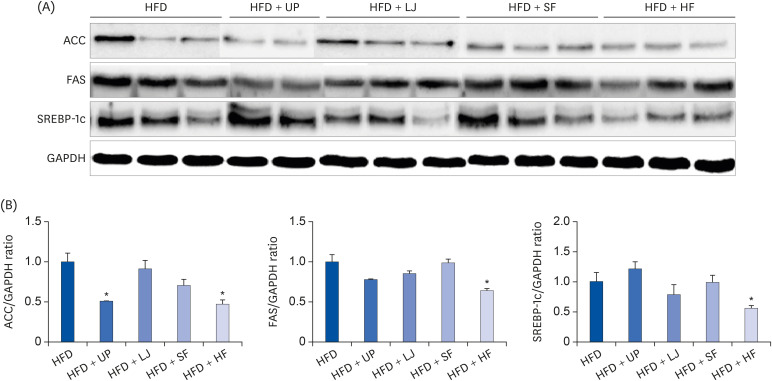 | Fig. 6Effects of lipogenesis-related proteins via seaweed supplementation in the livers of diet-induced obese mice. (A) Representative immunoblot analysis of ACC, FAS, SREBP-1c, and GAPDH in the liver, (B) Intensities of ACC, FAS, and SREBP-1c were normalized to GAPDH. Values are the mean ± standard error of the mean. An asterisk indicates a significant difference compared to the HFD group (P < 0.05).UP, Undaria pinnatifida; LJ, Laminaria japonica; SF, Sargassum fulvellum; HF, Hizikia fusiforme; ACC, acetyl-CoA carboxylase; FAS, fatty acid synthase; SREBP-1c, sterol regulatory element-binding protein-1c; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HFD, high fat diet.
|




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download