INTRODUCTION
In patients with tachycardia-bradycardia syndrome (TBS), paroxysmal atrial fibrillation (AF) often results in sinus pause after the termination of tachycardia. TBS is difficult to treat with antiarrhythmic agents alone because control of tachycardia may lead to aggravation of bradycardia.
1) Implantation of a permanent pacemaker (PM) with antiarrhythmic agents has been the traditional treatment for TBS. On the other hand, radiofrequency catheter ablation (RFCA) has emerged as an alternative treatment strategy for TBS,
2) with reportedly favorable mid-term results. In a recent study, sinus rhythm maintenance was remarkably higher in the RFCA group followed less than 2 years (83.7% vs 21.1% in the PM group, p<0.001) and most successful RFCA patients (95.3%) no longer needed a pacemaker.
3)4) A recent study reported that 8–13% of the patients with TBS with AF followed permanent PM insertion after receiving RFCA, and that 30% or more of patients with TBS with AF continued AF after permanent PM was inserted.
5)6)7) However, recurrence of AF has been reported high after catheter ablation, and data on the long-term clinical follow-up (over 3 years) of patients with TBS after RFCA are sparse. We aimed to investigate the long-term clinical outcomes of RFCA and compare the efficacy and safety of RFCA with PM implantation in patients with TBS.
Go to :

METHODS
Study patients
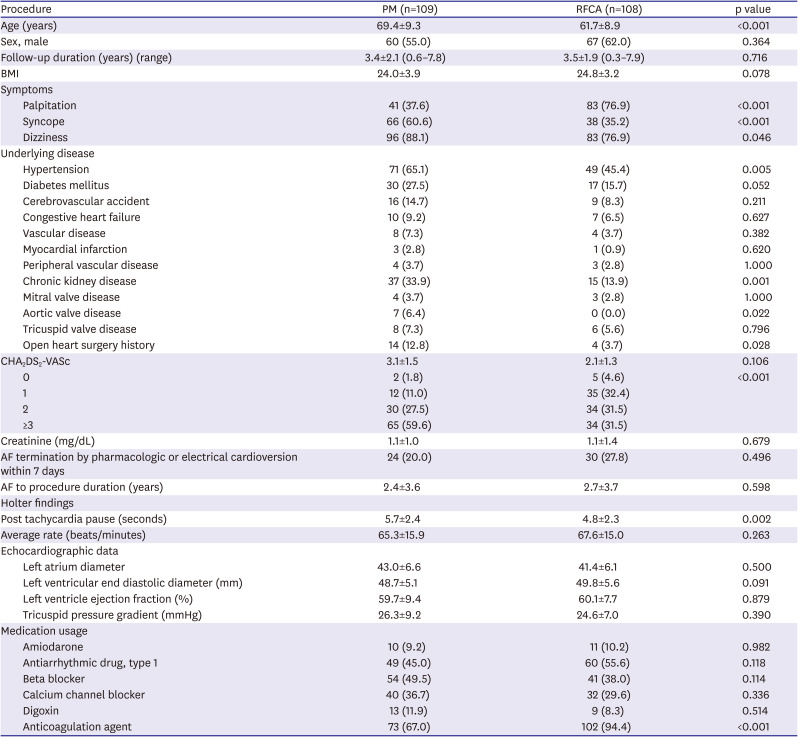
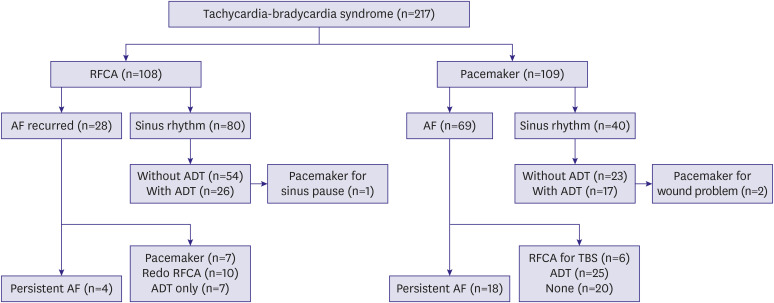
We reviewed the medical records of 217 patients with TBS with AF who were referred to Asan Medical Center between January 2010 and December 2017 and underwent either RFCA or PM implantation. TBS was defined as prolonged sinus pause (≥3 seconds) after AF termination on telemetry or Holter monitoring. The patients who already had PMs were excluded. RFCA or PM implantation was performed at the physician's discretion. The patients were divided into 2 groups according to the initial invasive treatment: RFCA (RFCA group) or PM implantation with antiarrhythmic drug therapy (PM group).
Catheter ablation for AF
RFCA for AF was performed in 108 patients with medically intractable AF and sinus pauses. RFCA was preferred as a first-line invasive treatment when AF symptoms were more evident than bradycardia symptoms. After discontinuation of all antiarrhythmic agents for at least 5 half-lives, an electrophysiological study was performed in the fasting state, typically under sedation. Patients taking amiodarone were instructed to discontinue medication 1 month prior to the procedure. A 7F Duodecapolar catheter was introduced percutaneously through the left femoral vein and placed in the right atrium in the coronary sinus region. Two 8F sheaths (SL1, St. Jude Medical, Inc., Saint Paul, MN, USA) were introduced into the LA using a modified Brockenbrough technique. After transseptal puncture, intravenous heparin was injected as a bolus (80 IU/Kg) and intermittently (1,000–2,000 IU/hour) to maintain an activated clotting time >300 seconds. In addition, heparinized saline was continuously infused through the transseptal sheath (3 mL/min) to avoid formation of thrombi or air embolisms. The infusion rate was increased to 17 mL/min (≤30 W) or 30 mL/min (>30 W) during radiofrequency application.
Multiple electrocardiographic leads (leads I, aVF and V1, filtered between 0.05 to 100 Hz) and intracardiac bipolar electrograms (filtered between 30 to 500 Hz) were simultaneously displayed and recorded on a digital electrophysiologic recording system (CardioLab®, Prucka Eng., Houston, TX, USA). The stimuli were delivered using a programmable digital stimulator (DTU-215; Bloom & Associates, Reading, PA, USA) at twice the diastolic threshold with a 2 ms pulse width for conventional pacing and at 10 to 20 mA for high output pacing. Conventional fluoroscopic mapping or 3-dimentional mapping was used.
Circumferential pulmonary vein isolation
The extent type of ablation lesion were determined at the physician's discretion. Usually, circumferential pulmonary vein (PV) isolation was performed 5–10 mm outside the PV ostia, with isolation of 2 ipsilateral veins in one circumferential lesion. In some cases, segmental ostial ablation of PV was done. Ablation in the left atrium was performed using an irrigated catheter with a target temperature of 43°C, maximal power of 30 W, and an infusion rate of 17 mL/min. Power was decreased to 25 W in the posterior left atrium and temporarily increased to 35–40 W in an anterior circumference when resistant to RF application. The end-point of the PV isolation was elimination or dissociation of the PV potentials. An ostial or carinal touch-up was additionally performed if PV potentials remained after circumferential PV isolation without an obvious gap along the line. Ablation at the PV ostia and within the coronary sinus was performed at 15–25 W. Energy was applied for 20 to 60 seconds at each point until significant (>80%) reduction of antral bipolar electrogram amplitude or disappearance of the sharp component of the bipolar electrogram. Three-dimensional mapping was performed using Carto XP® or EnSite® systems. After PV isolation, post-procedure AF induction test was performed routinely using isoproterenol. When cavotricuspid isthmus (CTI) flutter was induced, CTI ablation was done for bidirectional block. In addition, if AF was induced, CTI ablation was added as a part of defragmentation. Other linear ablation and complex fractionated atrial electrogram ablation were also performed as a routine procedure if AF was induced.
Pacemaker implantation
PM implantation was performed using standard technique according to accepted clinical guidelines
8)9)10)11) via the subclavian or axillary vein under local anesthesia with sedative drugs. All patients in the PM group received a dual-chamber PM that was programmed to rate-modulated (DDDR) mode. At implantation, bipolar atrial and ventricular electrodes were positioned and conventional measurements were performed in order to achieve satisfactory sensing values and stimulation thresholds. The lower rate limit was programmed at 60 beats per minute (bpm) and maximal tracking was programmed at 130 bpm. All the patients in the PM group were evaluated at discharge and at the first outpatient visit 30 days after the procedure to assess the sensing values and stimulation thresholds of atrial and ventricular leads and procedure-related complications.
Clinical follow-up
In the RFCA group, all the patients received 24-hour Holter monitoring the day after the procedure. Patients were seen in the outpatient clinic at 1, 3, and 6 months and every 6months thereafter. Twelve-lead electrocardiograms and ambulatory 24-hour Holter monitoring were checked on each visit. If patients complained of symptoms suggestive of recurrent AF, they received additional 24-hour Holter monitoring or a cardiac event recorder to verify the cause of their symptoms. Recurrence of atrial tachyarrhythmia (ATA) was defined in case it lasted at least 30 seconds after a 3-month blanking period. In the PM group, follow-up visits occurred 1, 6, and 12 months after implantation and then every 12 months for device interrogation.
Clinical outcomes were evaluated in each treatment group, including maintenance of sinus rhythm, additional procedure or crossover. The composite of cardiovascular rehospitalization and death, cerebral infarction, and myocardial infarction were also evaluated. Additional procedure was defined as a repeat of the same procedure or crossover treatment (for example, PM implantation after RFCA, or vice versa).
Statistical analysis
All continuous variables are presented as the mean±standard deviation and categorical variables are expressed as number (%). Continuous variables were compared using the t-test or the Mann-Whitney U test, and categorical variables were compared using the χ2 or Fisher's exact tests. Primary composite endpoints were calculated by the Kaplan-Meier method and were compared with the log-rank test. Multivariable logistic regression analysis was performed to compare each clinical outcome between 2 groups. The following clinical factors, echocardiographic parameters and drug history were selected for multivariable analysis: age, gender, body mass index, hypertension, diabetes, cerebrovascular accident, congestive heart failure, myocardial infarction history, chronic kidney disease, open heart surgery history, CHA2DS2-VASc score, left atrium diameter, left ventricle end diastolic diameter, pulmonary hypertension, antiarrhythmic agent, anticoagulation. All p values were 2-sided and a p value <0.05 was considered statistically significant. All statistical analyses were performed using SPSS version 18.0 for Window (SPSS Inc, Chicago, IL, USA).
Go to :

DISCUSSION
In this study of the role of catheter ablation involving are relatively large number of TBS patients than previous study, we first report the long-term arrhythmia recurrence rate, the rate of conversion to persistent AF, crossover to the opposite procedure, and the hard outcomes of the ablation strategy; namely, death, stroke, and myocardial infarction.
TBS is characterized by sinus node dysfunction or prolonged sinus pauses after termination of atrial tachyarrhythmias.
1) Several studies have described irreversible histologic changes such as fatty infiltration and extensive fibrosis of the sinoatrial node or surrounding junctional regions.
2)12) In this regard, treatment of TBS has generally emphasized control of the bradycardia-related symptoms associated with sinus node dysfunction. However, it has also been shown that the electro-anatomic changes in the sinus node are reversible after control of AF,
3) and successful catheter ablation of AF resolves the clinical manifestation of sinus node dysfunction in TBS patients.
13)14)15) This has led to the emergence of catheter ablation of AF as an important treatment option for patients with TBS.
Despite these positive clinical outcomes, concerns have been raised over the high long-term recurrence rate after AF ablation and the possibility of subsequent PM implantation. Although the results of previous studies are highly variable and heterogeneous,
4)16)17)18) a meta-analysis of 19 observational studies showed that the recurrence rate was as high as 53% with a single procedure.
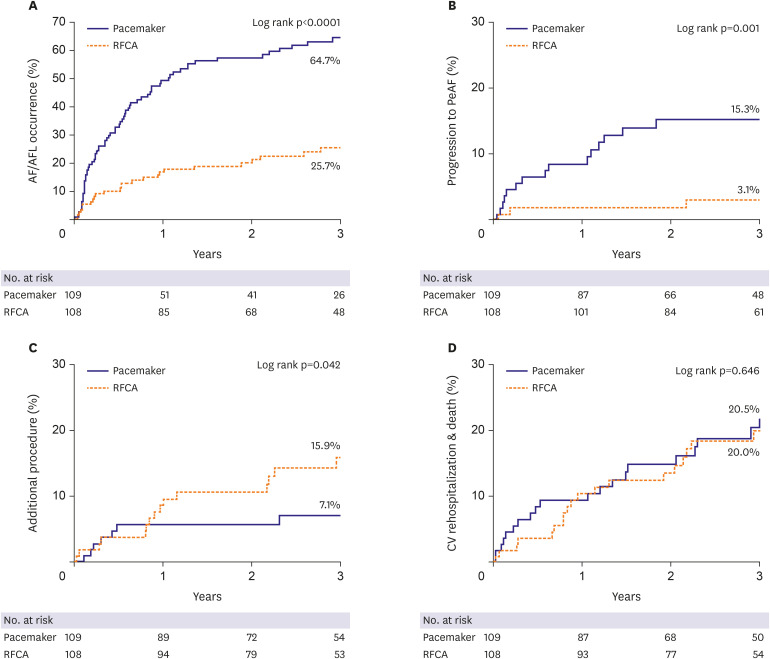
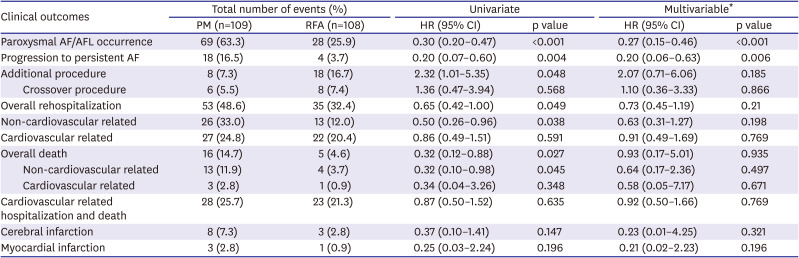
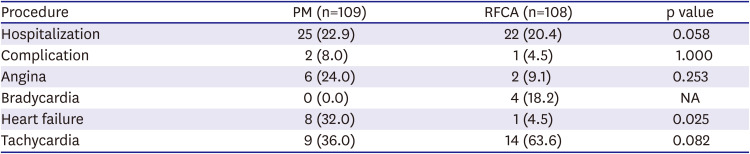
3) However, our study showed that during a long-term follow-up of over 3 years, AF recurrence after RFCA was relatively low (25.9%), and the proportion of patients who progressed to persistent AF was very low (3.7%). Moreover, the requirement for an additional procedure during long-term follow-up was small (16.7%), and very few patients required permanent PM implantation (7.4%) (
Table 2).
Although antiarrhythmic drug was used properly in the PM group, AF or AFL recurred in the majority of patients (63.3%), and paroxysmal AF converted to a persistent form in many (16.5%) (
Table 2). Several studies have confirmed the inadequacy of antiarrhythmic drugs for the maintenance of sinus rhythm,
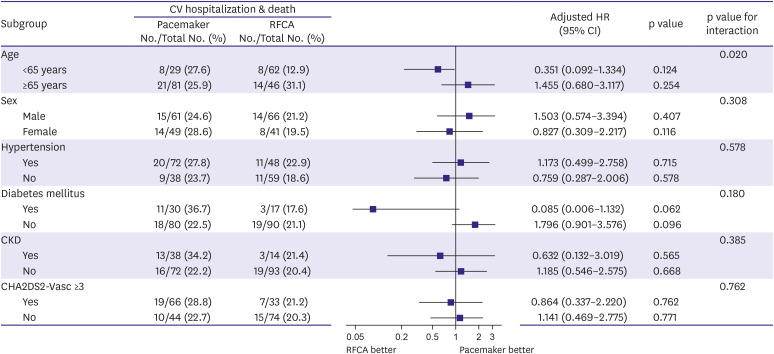
17)19) and AF burden is a known risk factor for stroke and congestive heart failure in patients with paroxysmal AF. Although our study did not show a significant difference, the incidence of tachycardia-related heart failure admission and occurrence of ischemic stroke were numerically higher in the PM group (
Tables 2 and
3). PM implantation appears to be a good treatment option for patients with a history of severe syncopal attacks and minimal tachycardia-related symptoms. However, tachycardia-related morbidity, such as aggravated heart failure, cerebral infarction, or underlying comorbidities should be considered.
It is difficult to compare the effectiveness and safety of RF ablation and PM implantation as first-line treatment strategies for patients with TBS. As demonstrated in our study, curative therapy of AF using RF ablation appears to be an effective and safe alternative to permanent PM implantation in the long-term. After successful RF ablation, sinus rhythm was better maintained over a prolonged period of time (>3 years) and reversion to persistent AF was less frequent than with PM implantation. Moreover, additional procedures and procedure crossover were similar in both groups, and PM implantation was required in only a small number of RFCA patients (7.4%) during the 3.5 years follow-up period. Recent study showed that in TBS patients, long pause on termination of AF predicts the need to implant a permanent pacemaker after catheter ablation.
20) Consistent with previous studies showing that patients undergoing successful catheter ablation have lower morbidity and mortality and higher quality of life,
21)22) our study also demonstrated that RF ablation is an effective and safe procedure in patients with TBS.
Assuming that RF ablation and PM implantation are equally effective and complementary for TBS treatment, selection between the treatment options should depend on the patient's condition and disease progression. RF ablation maybe more appropriate for patients with less structural change in the sinus node due to shorter duration of AF and a greater likelihood of restoration of sinus node function, relatively younger patients, patients with fewer comorbidities, and patients with palpitation as the chief concern. Patients with a lower chance to restore sinus node function and more structural change in the sinus node due to longer duration of AF, relatively older patients, patients with more comorbidities, and patients with dizziness or syncope as the chief concerns are more likely to benefit from PM implantation.
First, our study is a retrospective analysis from a single center and includes a relatively small number of patients. Thus, the findings must be validated in a prospective randomized study. Second, the selection of RFCA vs. PM implant was left to the physician's discretion. Therefore, patients with higher risk or morbidity may have been more likely to receive a PM implant. In our study, patients receiving a PM implant were more likely to be older and have bradycardia-related symptoms such as syncope or dizziness and have longer post tachycardia pause. There might be a selection bias in this study. Therefore, the results of our study might not apply to all patients with paroxysmal AF-related TBS. Third, after the procedure, the method of confirming AF/AFL recurrence was different by PM interrogation in the PM group and Holter in the RFCA group. The recurrence rate of AF/AFL might have been overestimated in the PM group. However, the rate of progression to persistent AF was not biased by the detection methods. Forth, CTI ablation in the RFCA group was performed in a rather large number of patients with 72%. The more frequent CTI ablation in RFCA group have influenced the low rate of occurrence of atrial tachycardia during follow-up. Fifth, only 45.5% of the pacemaker group had received anti-arrhythmic drug. Mild or no symptom of AF in this group might have affected the low prescription rate in this group. This has the potential to increase the incidence of atrial tachycardia in the PM group, so this can be one of source of bias in this study. Finally, AF recurrence was confirmed by ECG, Holter monitoring, and event recorder, and could have been underestimated, especially in patients with asymptomatic episodes.
In patients with prolonged sinus pause after AF termination, RF ablation of AF is an effective alternative treatment strategy as PM implantation. Catheter ablation of AF is related to stable long-term sinus rhythm maintenance and PM implantation does not need to be considered in most cases after successful ablation. The composite outcome of cardiovascular hospitalization and death is similar between patients undergoing RFCA and PM.
Go to :






 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download