INTRODUCTION
Chronic activation of the renin-angiotensin-aldosterone system (RAAS) plays an important role in the pathogenesis of cardiovascular diseases, and inhibition of RAAS reduces cardiovascular mortality and morbidity across a wide spectrum of cardiovascular disorders.
1)2) Specifically, increased angiotensin II levels after myocardial infarction (MI) exert adverse cardiovascular effects through vasoconstriction, fluid retention, sympathetic nerve system activation, platelet activation, and myocardial remodeling.
3)4)
RAAS blockade is accomplished therapeutically by two main groups of medications that have different pharmacologic actions: angiotensin converting enzyme inhibitors (ACEIs) and angiotensin II receptor blockers (ARBs).
1)2) ACEIs suppress not only the production of angiotensin, but also prevent the breakdown of bradykinin, thereby inducing additional cardioprotective effects. In contrast, ARBs reduce RAAS activity by selective inhibition of angiotensin II receptors.
Use of ACEIs after MI has been demonstrated to slow the progression of heart failure and reduce the occurrence of cardiovascular events. International guidelines recommend early use of ACEIs after an acute myocardial infarction (AMI), especially in patients with heart failure, hypertension, diabetes, or ST segment elevation MI.
5)6)7) However, ACEIs may produce cough, altered taste, or rash leading to dose reduction or permanent discontinuation of the medication, and drug discontinuation is more frequent with these drugs than with ARBs. ACEI intolerance is especially common in Asians, occurring in up to 20% of patients.
8)
ARBs are currently recommended for patients intolerant to ACEI therapy.
9)10) In two randomized controlled trials, ARBs and ACEIs exhibited similar efficacy for the prevention of death and cardiovascular morbidities after AMI.
11)12) However, clinical evidence in support of the use of ARBs in patients with AMI remains limited. In the present study, we used claims data from the Korean National Health Insurance Service (KNHIS) to compare clinical outcomes in real-world practice between the use of ARBs or ACEIs after AMI in patients who underwent percutaneous coronary intervention (PCI) with stent insertion.
METHODS
Study population
This is a retrospective cohort study using claims data from KNHIS, which is the sole insurer of the Korean national health insurance program. KNHIS operates a medical claims database that includes diagnoses, treatments, pharmaceuticals, and procedures, as well as personal data, such as age, sex, residential area, and date of death. All medical service providers and people seeking medical services in Korea are required by national acts to join the KNHIS insurance program. Therefore, the KNHIS database covers almost all medical services performed in the Korean population since 2002. The database is based on the Korean Standard Classification of Disease-7 coding system, which is compatible with the International Classification of Diseases-10 coding system.
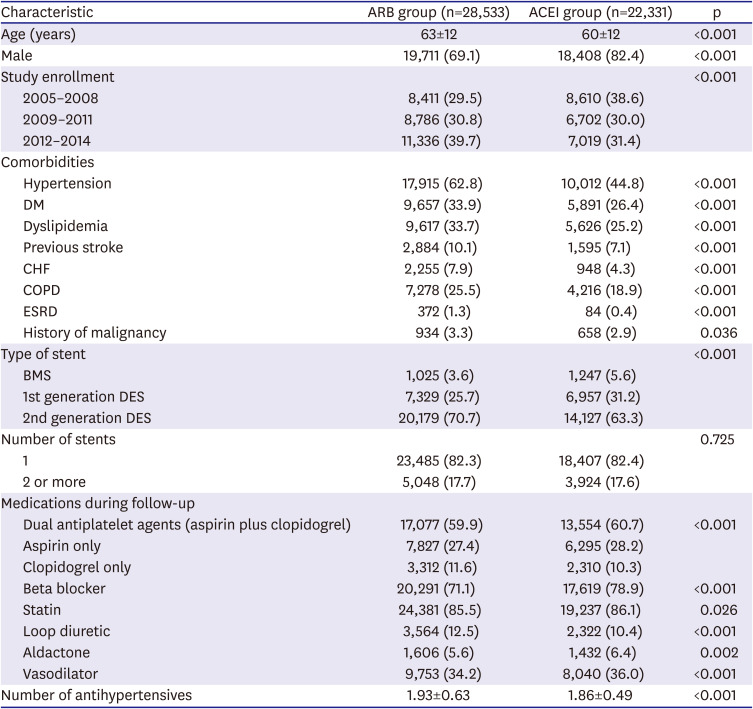
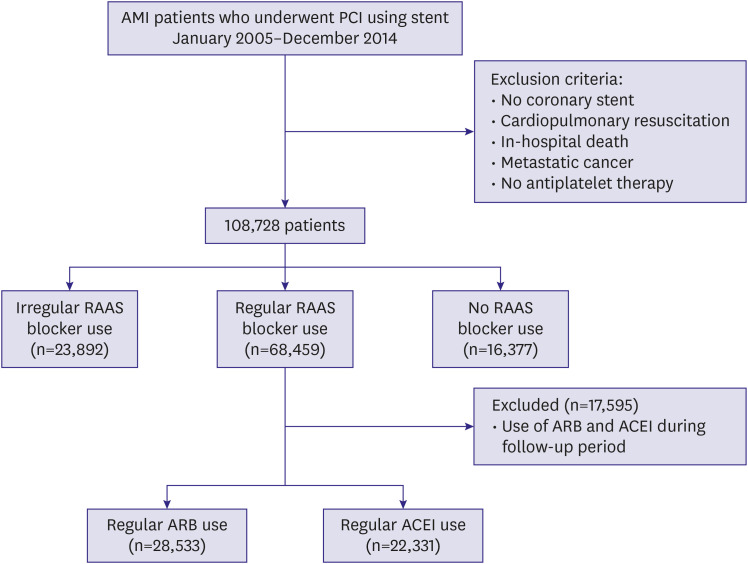
For the present study, we included all patients in the KNHIS database who underwent PCI using a bare metal stent or drug-eluting stent (DES) from 2005 to 2014 while admitted to a hospital under new diagnosis of AMI (diagnostic codes: I21, I22, or I23). The exclusion criteria were coronary bypass surgery, PCI without stent insertion, cardiopulmonary resuscitation, in-hospital death during the index admission, or the presence of metastatic cancer. We also excluded patients who received no antiplatelet agents after the index PCI to minimize confounding factors. The study population was followed after stent implantation for 2 years or until the primary end point occurred.
Data collection and outcome assessments
Data were collected by analyzing diagnostic, prescription, and procedural codes in the claims database. We calculated the medication possession ratio (MPR) by dividing the sum of days for which a medication was prescribed by the number of follow-up days. We classified the study population as regular medication users (MPR ≥80%), irregular medication users (MPR 1%–79%), or non-users (MPR 0%).
13) Only regular users of the respective medications were included in the outcome comparisons between the ARB and ACEI groups. Patients receiving both ACEIs and ARBs and those who changed medications from 1 group to the other during follow-up were excluded from the clinical outcomes comparisons. We defined the index time point as the date of admission for the procedure.
The primary end point was major adverse cardiovascular event (MACE), defined as the composite of all-cause death, myocardial infarction, or stroke (all types). MI was considered to have recurred during follow-up if the main diagnosis of AMI (diagnostic codes: I21, I22, or I23) was combined with rehospitalization and coronary angiography (procedure code: HA670) or if a new diagnosis of sudden cardiac arrest (diagnostic code: I469) was confirmed. We adopted this narrow definition of AMI to avoid misdiagnosis of a previous MI as a new event during follow-up. Strokes included ischemic stroke, hemorrhagic stroke, or unknown type of stroke (diagnostic codes: I60, I61, I62, I63, or I64) that were confirmed with an imaging study (examination codes: HE101, 201, 135, 235, 236, 451, or 461) during hospitalization.
Statistical analysis
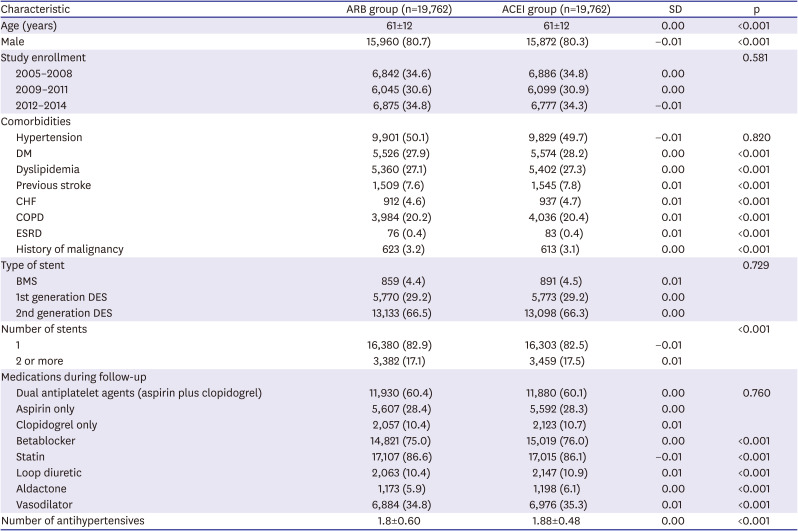
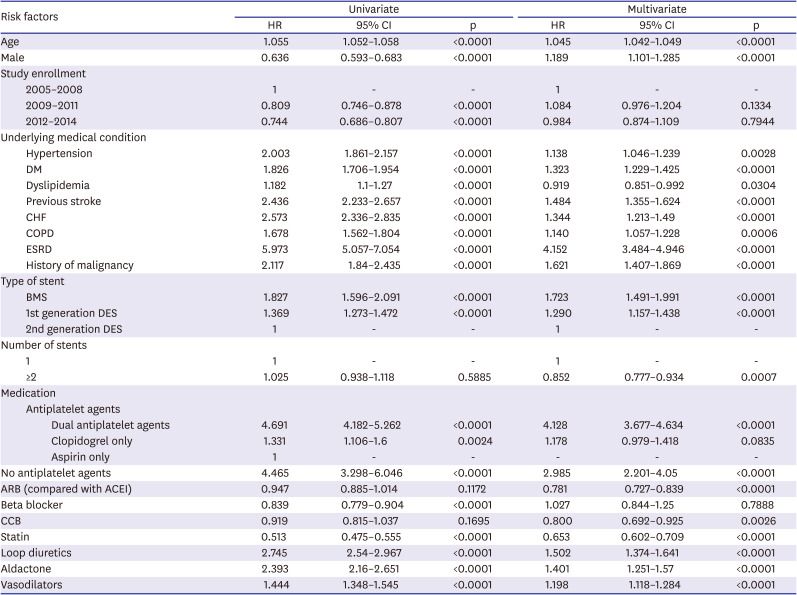
χ2 tests or Fisher's exact tests were used to compare categorical variables; Student's t-tests were used to compare continuous variables. Univariate and distributional analyses included measures of clinical outcomes. For propensity-score matching, we performed 1:1 case-control matching based on the propensity score with a hierarchical sequence until there were no more further matches. SAS logistic procedure code was used to create the propensity scores. The variables included in the propensity score matching were following confounding factors: age, sex, year of study enrollment, hypertension, diabetes mellitus, dyslipidemia, previous stroke, previous MI, heart failure, history of malignancy, stent type, number of stents, and in-hospital and follow-up medications (dual antiplatelet agents, beta blockers, calcium channel blockers, statins, loop diuretics, and spironolactone). Cox proportional-hazards regression analysis was used to assess the effects of ACEIs or ARBs as independent predictors of MACE. Statistical analysis was performed using SAS software (SAS Institute, Ver 9.1, Cary, NC, USA). The statistical significance level was set at p<0.05.
DISCUSSION
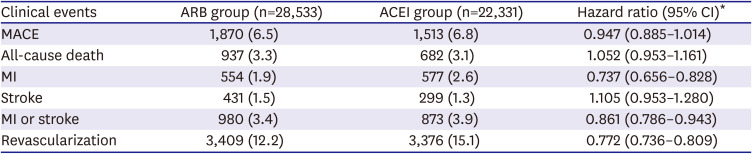
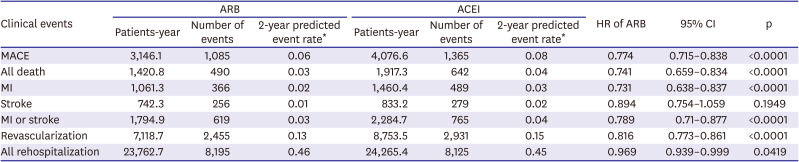
In this retrospective study using KNHIS claims data, we found that patients with AMI treated with PCI were more frequently prescribed ARBs than ACEIs. Furthermore, patients treated with ARBs had significantly lower rates of adverse clinical events, including MACE, all-cause death, MI, and revascularization, than those treated with ACEIs.
Although both ARBs and ACEIs modulate the RAAS, these drugs have different action mechanisms which may lead to different effects on cardiovascular outcomes in AMI patients. ACEIs not only block the conversion of angiotensin I to angiotensin II but also the degradation of bradykinin by inhibition of angiotensin-converting enzyme.
1)2) Thereby, ACEIs decrease the formation of angiotensin II, a potent vasopressor, but increase the level of bradykinin, a vasodilator. However, long-term treatment with ACEIs leads to a gradual rise of circulating angiotensin II concentration to pretreatment levels, a phenomenon described as “angiotensin II escape.”
14) Angiotensin II production by other enzymes in the tissues may be responsible for this phenomenon. In contrast, ARBs directly target on the angiotensin type 1 receptor (AT1R).
15) The selective action on the attenuated total internal reflection leads to unopposed stimulation of angiotensin type 2 receptor, which in turn counteracts the vasoconstrictive effects due to AT1R stimulation.
13) However, ARBs bypass the bradykinin pathway and may lack potential cardiovascular protective effects of bradykinin. On the other hand, ARBs do not induce cough or angioedema by accumulation of bradykinin.
16)
Recent meta-analyses demonstrated that ACEIs and ARBs have similar effectiveness for preventing major cardiovascular outcomes, such as AMI, stroke, and heart failure or hospitalization, although ACEIs are more effective for reducing total deaths and cardiovascular deaths.
17)18)19) However, only 2 randomized controlled trials have compared ARBs versus ACEIs for preventing mortality and morbidity after AMI.
11)12) The Optimal Trial in Myocardial Infarction with the Angiotensin II Antagonist Losartan (OPTIMAAL),
11) which compared losartan with captopril in patients with AMI and symptoms of heart failure or left ventricular dysfunction, found no significant differences between the 2 drugs for all-cause mortality and cardiovascular events, although there was a trend towards lower mortality in the captopril group. In the Valsartan in Acute Myocardial Infarction (VALIANT) trial,
12) which compared valsartan with captopril in patients with heart failure or left ventricular systolic dysfunction after AMI, valsartan was non-inferior to captopril with regard to total mortality and fatal or non-fatal adverse cardiovascular events. Based on these trials, current guidelines recommend ACEIs as first-choice treatment after AMI in patients with heart failure and/or left ventricular dysfunction, with ARBs considered an alternative for ACEI-intolerant patients.
9)10)20)21) However, these trials were conducted in the early 2000s; reperfusion therapy was performed in only approximately 10% and 30% of subjects in the OPTIMAAL
11) and VALIANT
12) trials, respectively; and the statin prescription rate was only 30%. Thus, these trials may not reflect contemporary clinical practice for the treatment of AMI.
In recent years, several retrospective cohort studies comparing ARB and ACEI use after AMI reported inconsistent results. Hara et al. found that ACEIs were associated with better survival than ARBs.
22) Similarly, Korean investigators such as Ann et al.
23) and Choi et al.
24) also demonstrated improved survival with ACEIs. By contrast, Lee et al.
25) reported that ARBs were superior to ACEIs for preventing in-hospital mortality and 12-month MACE, findings compatible with our study results. Other Korean data by Yang et al.
26) showed comparable effectiveness of ARBs and ACEIs. Unlike the randomized controlled trials, these cohort studies included patients both with and without heart failure or left ventricular dysfunction after AMI.
19)22)23)24)25)26) The percentages of patients with heart failure or Killip class II/IV in these cohort studies ranged from 2.2% to 18.1%. In the present study, approximately 6% of enrolled patients had congestive heart failure. Thus, the results of the cohort studies cannot be directly compared with those of the randomized control trials. A recent meta-analysis reported that ARBs effectively reduce cardiovascular mortality and morbidity in patients without heart failure; although studied in a non-AMI setting,
27) these findings are consistent with the results of our study. Previously, several studies reported increased cardiovascular events in patients treated with ARBs, suggesting the existence of an ARB MI paradox.
28) We did not observe an increased risk of cardiovascular events with ARB use in the present analysis.
Our study has several strengths. Firstly, it represents data from essentially all patients with an AMI treated with PCI (including stent insertion) in Korea between 2005 and 2014, which included >100,000 patients. This is the largest study population reported so far. Secondly, all clinical events during the follow-up period were captured; collecting data from the KNHIS database minimized loss of outcome information. Thirdly, our study differs from the previous studies in the study design. The previous studies categorized patient groups based on hospital discharge medication. However, in our study, the patient groups were analyzed based on the outpatient prescription for a 2-year follow-up period. Furthermore, this study accounted for treatment compliance by including only patients with an MPR ≥80% when comparing outcomes of ARBs versus ACEIs. No prior cohort study accounted for compliance in their analyses. Ortolani et al.
29) reported that poor adherence to ACEI or ARB prescription medication was associated with a 20% increased risk of recurrent AMI. We further excluded 17,595 patients who received both ACEIs and ARBs during the follow-up period. Among them, 84.3% changed the medication from an ACEI to an ARB. Thus, discharge medication may not represent the actual medication the patients take for long term. In the OPTIMAAL study, ARBs were significantly better tolerated than ACEIs, with fewer patients discontinuing study medication.
11) The relatively high rate of conversion from ACEI to ARB observed in our study likely reflects the previously reported higher rate of ACEI intolerance in Asians.
8)
Our study also has limitations. Firstly, this is a retrospective observational study. The possibility of bias cannot be excluded, despite the use of propensity score–matching to adjust for known potential confounding factors. Secondly, in this study, we included all patients who underwent PCI using stents under new diagnosis of MI. However, since cardiac biomarker or electrocardiogram data were not available, the accuracy of MI diagnosis remains uncertain. Furthermore, MI was considered to have recurred during follow-up if the diagnosis of MI was combined with rehospitalization and coronary angiography or if a new diagnosis of sudden cardiac arrest was confirmed. However, some of these patients might have been rehospitalized and have undergone coronary angiography for reasons other than recurred MI. Thus, the rate of recurred MI might have been overestimated. Thirdly, we were unable to obtain important laboratory, angiography or echocardiography data. Thus, we could not accurately determine cardiovascular disease status or heart failure severity, although we tried to adjust for left ventricular systolic dysfunction by identifying patients prescribed loop diuretics or spironolactone. In addition, there might have been also other confounding factors that were not included in the KNHIS database. Lastly, we were unable to collect information about the dosage of ACEIs or ARBs, which also may have impact on the clinical outcomes.
In conclusion, ARB use was associated with lower rates of MACE, MI, and revascularization than ACEIs in our retrospective analysis of a large, diverse group of patients with AMI treated with PCI in Korea. Further clinical trials are required to validate our findings.




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download