INTRODUCTION
Congenital heart disease (CHD) is one of the most common birth defects, with an incidence of 5–8 per 1,000 live births.
1) However, its incidence is 2 times higher in preterm than full-term infants.
2) In addition, the incidence of CHD reportedly increases to 20–40 per 1,000 live births in very low birth weight (VLBW) infants weighing less than 1,500 g.
3) The survival rate of VLBW infants is increasing due to advances in neonatal medicine; thus, the number of VLBW infants with CHD (CHD-VLBWs) is also expected to increase. These CHD-VLBWs are considered a unique patient group that has characteristics of both CHD patients and VLBW infants.
However, studies of CHD-VLBWs are insufficient to date. Past studies on preterm infants with CHD mainly included infants under 37 or 35 weeks of gestational age,
4) and most were based on insurance or big data rather than clinical data
5)6); thus, the derived information cannot aid clinicians in the clinical setting. Therefore, more specific information is needed to determine whether CHD-VLBWs develop more prematurity-associated diseases than VLBW infants, which factors are related with the disease in CHD-VLBWs, which patients have a worse prognosis in cyanotic CHD, and the surgery-related prognosis.
This study aimed to determine the morbidity, associated risk factors, and mortality of CHD-VLBWs using detailed clinical data obtained from a single institution.
Go to :

RESULTS
A total of 1,230 VLBW infants were born during the study. Among them, 1,050 were analyzed; 38 infants with simple secundum ASD without accompanying other heart defect, 31 who were transferred to other hospitals, 92 for whom insufficient medical records were available, and 19 with other congenital anomalies were omitted. Seventy-nine (7.5%) infants were diagnosed with CHD, of whom 58 had acyanotic CHD and 21 had cyanotic CHD (
Figure 1). The mean gestational age was 31.1±3.2 weeks and mean birth weight was 1,126.2±268.3 g; 50.6% were SGA.
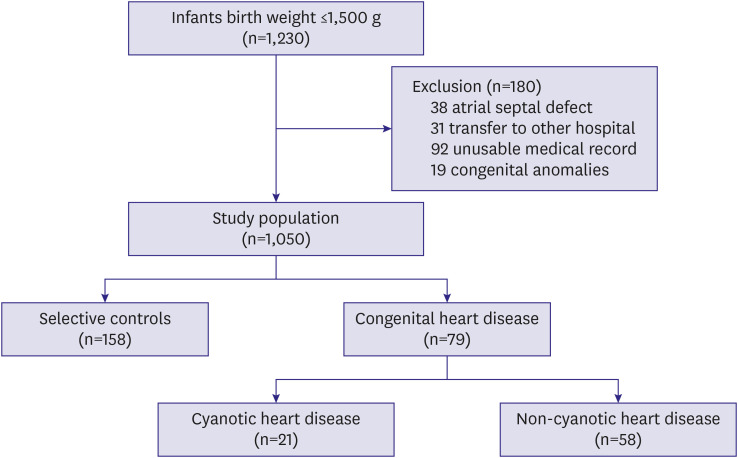 | Figure 1 Study population eligibility and enrollment.
|
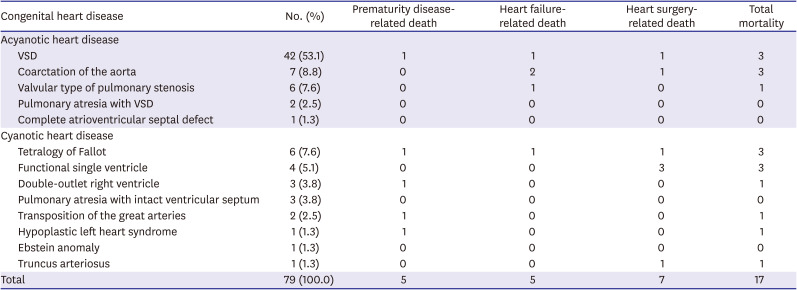
The most common CHD was ventricular septal defect (VSD; n = 42 [53.1%]), coarctation of the aorta (n = 7 [8.8%]), and tetralogy of Fallot (TOF; n = 6 [7.6%]) in order. Seventeen patients (21.5%) died, 10 (12.6%) of whom died preoperatively and the causes of death included 5 (6.3%) of prematurity-related diseases, 5 (6.3%) of heart failure, and 7 (8.9%) died after surgery, all of whom died of heart surgery-related diseases (
Table 1). Of the 7 post-operative deaths, 6 (85.7%) occurred in patients who previously underwent pulmonary artery banding (PAB) and 1 (14.3%) occurred in a patient after undergoing widening of the right ventricular outflow tract in TOF. Among the patients who underwent surgery, 6 underwent PAB, and all 6 died. Other patients who underwent central shunt procedures, pulmonary valvotomy, coarctoplasty, or VSD repair survived.
Table 1
Distribution of congenital heart disease by International Classification of Diseases, Version 9, Clinical Modification code
|
Congenital heart disease |
No. (%) |
Prematurity disease-related death |
Heart failure-related death |
Heart surgery-related death |
Total mortality |
|
Acyanotic heart disease |
|
|
|
|
|
|
VSD |
42 (53.1) |
1 |
1 |
1 |
3 |
|
Coarctation of the aorta |
7 (8.8) |
0 |
2 |
1 |
3 |
|
Valvular type of pulmonary stenosis |
6 (7.6) |
0 |
1 |
0 |
1 |
|
Pulmonary atresia with VSD |
2 (2.5) |
0 |
0 |
0 |
0 |
|
Complete atrioventricular septal defect |
1 (1.3) |
0 |
0 |
0 |
0 |
|
Cyanotic heart disease |
|
|
|
|
|
|
Tetralogy of Fallot |
6 (7.6) |
1 |
1 |
1 |
3 |
|
Functional single ventricle |
4 (5.1) |
0 |
0 |
3 |
3 |
|
Double-outlet right ventricle |
3 (3.8) |
1 |
0 |
0 |
1 |
|
Pulmonary atresia with intact ventricular septum |
3 (3.8) |
0 |
0 |
0 |
0 |
|
Transposition of the great arteries |
2 (2.5) |
1 |
0 |
0 |
1 |
|
Hypoplastic left heart syndrome |
1 (1.3) |
1 |
0 |
0 |
1 |
|
Ebstein anomaly |
1 (1.3) |
0 |
0 |
0 |
0 |
|
Truncus arteriosus |
1 (1.3) |
0 |
0 |
1 |
1 |
|
Total |
79 (100.0) |
5 |
5 |
7 |
17 |

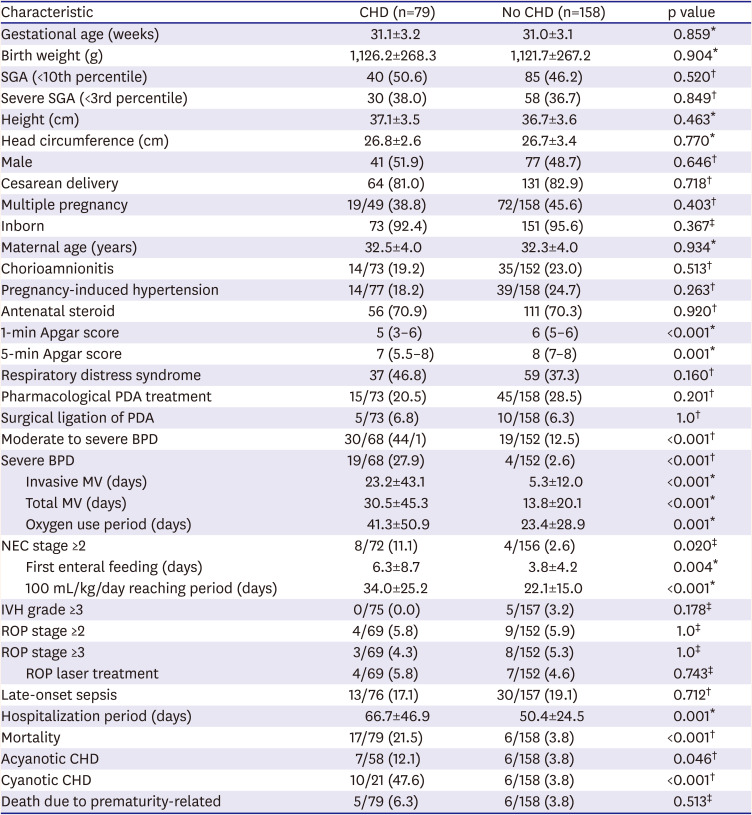
Clinical characteristics and neonatal outcomes were compared between the CHD-VLBWs group and the matched control group. Although there was no statistically significant intergroup difference in demographic variables, the incidence of moderate to severe BPD and severe BPD was higher in CHD-VLBWs than in controls. There were no statistically significant intergroup differences in surgical and drug treatment for PDA that could affect the development of BPD. NEC occurred more frequently in the CHD-VLBWs. The time to reach a feeding up to 100 mL/kg/day and first enteral feeding days were longer in the CHD-VLBWs than controls regardless of breast milk or preterm formula. The CHD-VLBWs had higher mortality and prolonged hospitalization rates. It has also been shown to increase mortality in both acyanotic and cyanotic CHD patients. No significant difference in mortality due to prematurity-related diseases was found according to the presence or absence of CHD. However, IVH, ROP, and LOS did not differ significantly between the 2 groups (
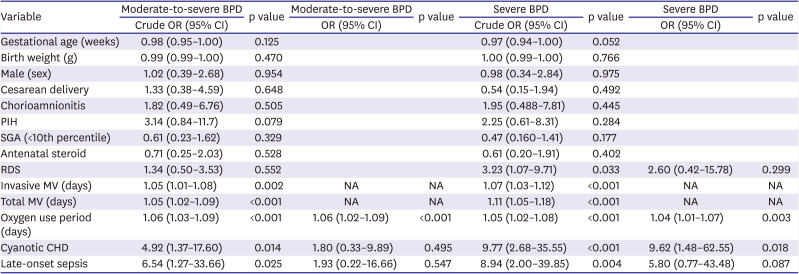
Table 2). Through the analysis to determine the factors that increase the risk of BPD in CHD-VLBWs, mechanical ventilation duration, oxygen supplementation duration, cyanotic heart disease, and LOS were identified as risk factors. Among these factors, as the durations of mechanical ventilation and oxygen supplementation showed multicollinearity, oxygen supplementation was chosen and adjusted in the multiple regression analysis. After the relevant factors were adjusted, the oxygen supplementation duration was found to be a risk factor for the development of moderate-to-severe BPD and severe BPD, and cyanotic CHD was a risk factor of severe BPD (
Table 3). On multivariate analysis, the incidence of NEC was associated with CHD in whole cases and not with other factors, such as first enteral feeding day or advancement in feeding to 100 mL/kg/day. To identify feeding advancing factors in connection with the development of NEC in CHD-VLBWs, early enteral feeding and the beginning of the first feeding were analyzed; however, no statistically significant association with NEC was detected (
Table 4).
Table 2
Comparing characteristics, morbidity and mortality between CHD and no CHD groups
|
Characteristic |
CHD (n=79) |
No CHD (n=158) |
p value |
|
Gestational age (weeks) |
31.1±3.2 |
31.0±3.1 |
0.859*
|
|
Birth weight (g) |
1,126.2±268.3 |
1,121.7±267.2 |
0.904*
|
|
SGA (<10th percentile) |
40 (50.6) |
85 (46.2) |
0.520†
|
|
Severe SGA (<3rd percentile) |
30 (38.0) |
58 (36.7) |
0.849†
|
|
Height (cm) |
37.1±3.5 |
36.7±3.6 |
0.463*
|
|
Head circumference (cm) |
26.8±2.6 |
26.7±3.4 |
0.770*
|
|
Male |
41 (51.9) |
77 (48.7) |
0.646†
|
|
Cesarean delivery |
64 (81.0) |
131 (82.9) |
0.718†
|
|
Multiple pregnancy |
19/49 (38.8) |
72/158 (45.6) |
0.403†
|
|
Inborn |
73 (92.4) |
151 (95.6) |
0.367‡
|
|
Maternal age (years) |
32.5±4.0 |
32.3±4.0 |
0.934*
|
|
Chorioamnionitis |
14/73 (19.2) |
35/152 (23.0) |
0.513†
|
|
Pregnancy-induced hypertension |
14/77 (18.2) |
39/158 (24.7) |
0.263†
|
|
Antenatal steroid |
56 (70.9) |
111 (70.3) |
0.920†
|
|
1-min Apgar score |
5 (3–6) |
6 (5–6) |
<0.001*
|
|
5-min Apgar score |
7 (5.5–8) |
8 (7–8) |
0.001*
|
|
Respiratory distress syndrome |
37 (46.8) |
59 (37.3) |
0.160†
|
|
Pharmacological PDA treatment |
15/73 (20.5) |
45/158 (28.5) |
0.201†
|
|
Surgical ligation of PDA |
5/73 (6.8) |
10/158 (6.3) |
1.0†
|
|
Moderate to severe BPD |
30/68 (44/1) |
19/152 (12.5) |
<0.001†
|
|
Severe BPD |
19/68 (27.9) |
4/152 (2.6) |
<0.001†
|
|
Invasive MV (days) |
23.2±43.1 |
5.3±12.0 |
<0.001*
|
|
Total MV (days) |
30.5±45.3 |
13.8±20.1 |
<0.001*
|
|
Oxygen use period (days) |
41.3±50.9 |
23.4±28.9 |
0.001*
|
|
NEC stage ≥2 |
8/72 (11.1) |
4/156 (2.6) |
0.020‡
|
|
First enteral feeding (days) |
6.3±8.7 |
3.8±4.2 |
0.004*
|
|
100 mL/kg/day reaching period (days) |
34.0±25.2 |
22.1±15.0 |
<0.001*
|
|
IVH grade ≥3 |
0/75 (0.0) |
5/157 (3.2) |
0.178‡
|
|
ROP stage ≥2 |
4/69 (5.8) |
9/152 (5.9) |
1.0‡
|
|
ROP stage ≥3 |
3/69 (4.3) |
8/152 (5.3) |
1.0‡
|
|
ROP laser treatment |
4/69 (5.8) |
7/152 (4.6) |
0.743‡
|
|
Late-onset sepsis |
13/76 (17.1) |
30/157 (19.1) |
0.712†
|
|
Hospitalization period (days) |
66.7±46.9 |
50.4±24.5 |
0.001*
|
|
Mortality |
17/79 (21.5) |
6/158 (3.8) |
<0.001†
|
|
Acyanotic CHD |
7/58 (12.1) |
6/158 (3.8) |
0.046†
|
|
Cyanotic CHD |
10/21 (47.6) |
6/158 (3.8) |
<0.001†
|
|
Death due to prematurity-related |
5/79 (6.3) |
6/158 (3.8) |
0.513‡
|

Table 3
Multiple regression analysis of the BPD-related factors in very low birth weight infants with CHD
|
Variable |
Moderate-to-severe BPD |
p value |
Moderate-to-severe BPD |
p value |
Severe BPD |
p value |
Severe BPD |
p value |
|
Crude OR (95% CI) |
OR (95% CI) |
Crude OR (95% CI) |
OR (95% CI) |
|
Gestational age (weeks) |
0.98 (0.95–1.00) |
0.125 |
|
|
0.97 (0.94–1.00) |
0.052 |
|
|
|
Birth weight (g) |
0.99 (0.99–1.00) |
0.470 |
|
|
1.00 (0.99–1.00) |
0.766 |
|
|
|
Male (sex) |
1.02 (0.39–2.68) |
0.954 |
|
|
0.98 (0.34–2.84) |
0.975 |
|
|
|
Cesarean delivery |
1.33 (0.38–4.59) |
0.648 |
|
|
0.54 (0.15–1.94) |
0.492 |
|
|
|
Chorioamnionitis |
1.82 (0.49–6.76) |
0.505 |
|
|
1.95 (0.488–7.81) |
0.445 |
|
|
|
PIH |
3.14 (0.84–11.7) |
0.079 |
|
|
2.25 (0.61–8.31) |
0.284 |
|
|
|
SGA (<10th percentile) |
0.61 (0.23–1.62) |
0.329 |
|
|
0.47 (0.160–1.41) |
0.177 |
|
|
|
Antenatal steroid |
0.71 (0.25–2.03) |
0.528 |
|
|
0.61 (0.20–1.91) |
0.402 |
|
|
|
RDS |
1.34 (0.50–3.53) |
0.552 |
|
|
3.23 (1.07–9.71) |
0.033 |
2.60 (0.42–15.78) |
0.299 |
|
Invasive MV (days) |
1.05 (1.01–1.08) |
0.002 |
NA |
NA |
1.07 (1.03–1.12) |
<0.001 |
NA |
NA |
|
Total MV (days) |
1.05 (1.02–1.09) |
<0.001 |
NA |
NA |
1.11 (1.05–1.18) |
<0.001 |
NA |
NA |
|
Oxygen use period (days) |
1.06 (1.03–1.09) |
<0.001 |
1.06 (1.02–1.09) |
<0.001 |
1.05 (1.02–1.08) |
<0.001 |
1.04 (1.01–1.07) |
0.003 |
|
Cyanotic CHD |
4.92 (1.37–17.60) |
0.014 |
1.80 (0.33–9.89) |
0.495 |
9.77 (2.68–35.55) |
<0.001 |
9.62 (1.48–62.55) |
0.018 |
|
Late-onset sepsis |
6.54 (1.27–33.66) |
0.025 |
1.93 (0.22–16.66) |
0.547 |
8.94 (2.00–39.85) |
0.004 |
5.80 (0.77–43.48) |
0.087 |

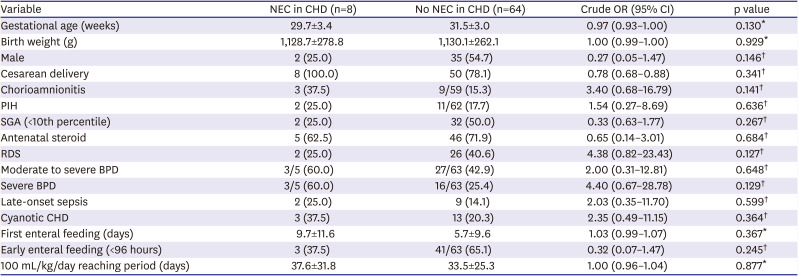
Table 4
NEC-related factors in very low birth weight infants with CHD
|
Variable |
NEC in CHD (n=8) |
No NEC in CHD (n=64) |
Crude OR (95% CI) |
p value |
|
Gestational age (weeks) |
29.7±3.4 |
31.5±3.0 |
0.97 (0.93–1.00) |
0.130*
|
|
Birth weight (g) |
1,128.7±278.8 |
1,130.1±262.1 |
1.00 (0.99–1.00) |
0.929*
|
|
Male |
2 (25.0) |
35 (54.7) |
0.27 (0.05–1.47) |
0.146†
|
|
Cesarean delivery |
8 (100.0) |
50 (78.1) |
0.78 (0.68–0.88) |
0.341†
|
|
Chorioamnionitis |
3 (37.5) |
9/59 (15.3) |
3.40 (0.68–16.79) |
0.141†
|
|
PIH |
2 (25.0) |
11/62 (17.7) |
1.54 (0.27–8.69) |
0.636†
|
|
SGA (<10th percentile) |
2 (25.0) |
32 (50.0) |
0.33 (0.63–1.77) |
0.267†
|
|
Antenatal steroid |
5 (62.5) |
46 (71.9) |
0.65 (0.14–3.01) |
0.684†
|
|
RDS |
2 (25.0) |
26 (40.6) |
4.38 (0.82–23.43) |
0.127†
|
|
Moderate to severe BPD |
3/5 (60.0) |
27/63 (42.9) |
2.00 (0.31–12.81) |
0.648†
|
|
Severe BPD |
3/5 (60.0) |
16/63 (25.4) |
4.40 (0.67–28.78) |
0.129†
|
|
Late-onset sepsis |
2 (25.0) |
9 (14.1) |
2.03 (0.35–11.70) |
0.599†
|
|
Cyanotic CHD |
3 (37.5) |
13 (20.3) |
2.35 (0.49–11.15) |
0.364†
|
|
First enteral feeding (days) |
9.7±11.6 |
5.7±9.6 |
1.03 (0.99–1.07) |
0.367*
|
|
Early enteral feeding (<96 hours) |
3 (37.5) |
41/63 (65.1) |
0.32 (0.07–1.47) |
0.245†
|
|
100 mL/kg/day reaching period (days) |
37.6±31.8 |
33.5±25.3 |
1.00 (0.96–1.04) |
0.877*
|

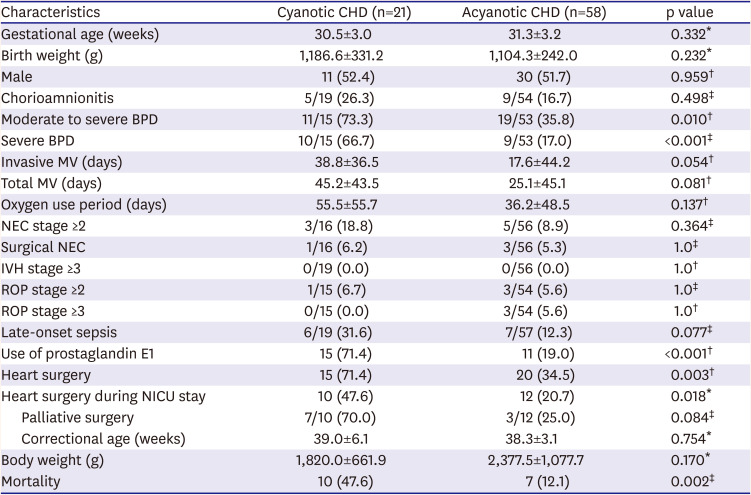
Of 79 CHD-VLBWs, there were 21 cases of cyanotic CHD. The most common cyanotic CHD was TOF (6 cases [28.5%]), functional single ventricle (4 cases [19.0%]), double-outlet right ventricle (3 cases [14.0%]), and pulmonary atresia with intact ventricle septum (3 cases, 14.0%). The incidence of moderate to severe BPD and severe BPD was higher in cyanotic CHD than acyanotic CHD. However, IVH, ROP, LOS, and NEC did not differ significantly between the 2 groups. Use of prostaglandin E1 was more common in the cyanotic CHD group. Cyanotic CHD-VLBWs underwent heart surgery more frequently than acyanotic CHD-VLBWs during hospitalization in the NICU and including after discharge, respectively (p=0.018, p=0.003). The mortality rate was also higher in cyanotic CHD-VLBWs (
Table 5).
Table 5
Characteristics, morbidities, and mortality between cyanotic and acyanotic CHD groups
|
Characteristics |
Cyanotic CHD (n=21) |
Acyanotic CHD (n=58) |
p value |
|
Gestational age (weeks) |
30.5±3.0 |
31.3±3.2 |
0.332*
|
|
Birth weight (g) |
1,186.6±331.2 |
1,104.3±242.0 |
0.232*
|
|
Male |
11 (52.4) |
30 (51.7) |
0.959†
|
|
Chorioamnionitis |
5/19 (26.3) |
9/54 (16.7) |
0.498‡
|
|
Moderate to severe BPD |
11/15 (73.3) |
19/53 (35.8) |
0.010†
|
|
Severe BPD |
10/15 (66.7) |
9/53 (17.0) |
<0.001‡
|
|
Invasive MV (days) |
38.8±36.5 |
17.6±44.2 |
0.054†
|
|
Total MV (days) |
45.2±43.5 |
25.1±45.1 |
0.081†
|
|
Oxygen use period (days) |
55.5±55.7 |
36.2±48.5 |
0.137†
|
|
NEC stage ≥2 |
3/16 (18.8) |
5/56 (8.9) |
0.364‡
|
|
Surgical NEC |
1/16 (6.2) |
3/56 (5.3) |
1.0‡
|
|
IVH stage ≥3 |
0/19 (0.0) |
0/56 (0.0) |
1.0†
|
|
ROP stage ≥2 |
1/15 (6.7) |
3/54 (5.6) |
1.0‡
|
|
ROP stage ≥3 |
0/15 (0.0) |
3/54 (5.6) |
1.0†
|
|
Late-onset sepsis |
6/19 (31.6) |
7/57 (12.3) |
0.077‡
|
|
Use of prostaglandin E1 |
15 (71.4) |
11 (19.0) |
<0.001†
|
|
Heart surgery |
15 (71.4) |
20 (34.5) |
0.003†
|
|
Heart surgery during NICU stay |
10 (47.6) |
12 (20.7) |
0.018*
|
|
Palliative surgery |
7/10 (70.0) |
3/12 (25.0) |
0.084‡
|
|
Correctional age (weeks) |
39.0±6.1 |
38.3±3.1 |
0.754*
|
|
Body weight (g) |
1,820.0±661.9 |
2,377.5±1,077.7 |
0.170*
|
|
Mortality |
10 (47.6) |
7 (12.1) |
0.002‡
|

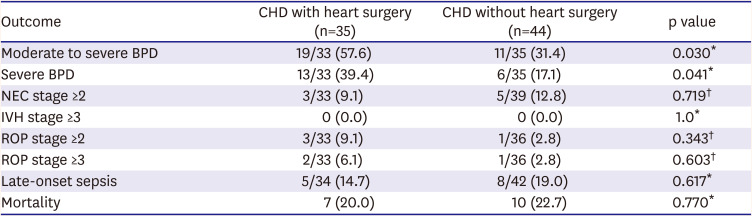
Heart surgery itself did not affect postoperative mortality (p=0.770). However, moderate to severe BPD and severe BPD were increased in CHD-VLBWs who underwent surgery (p=0.030, p=0.041, respectively). Other morbidities such as NEC, ROP, IVH, and LOS did not differ significantly between CHD-VLBWs who underwent surgery and those who did not (
Table 6).
Table 6
Characteristics, morbidity, and mortality of CHD patients who did versus did not undergo heart surgery
|
Outcome |
CHD with heart surgery (n=35) |
CHD without heart surgery (n=44) |
p value |
|
Moderate to severe BPD |
19/33 (57.6) |
11/35 (31.4) |
0.030*
|
|
Severe BPD |
13/33 (39.4) |
6/35 (17.1) |
0.041*
|
|
NEC stage ≥2 |
3/33 (9.1) |
5/39 (12.8) |
0.719†
|
|
IVH stage ≥3 |
0 (0.0) |
0 (0.0) |
1.0*
|
|
ROP stage ≥2 |
3/33 (9.1) |
1/36 (2.8) |
0.343†
|
|
ROP stage ≥3 |
2/33 (6.1) |
1/36 (2.8) |
0.603†
|
|
Late-onset sepsis |
5/34 (14.7) |
8/42 (19.0) |
0.617*
|
|
Mortality |
7 (20.0) |
10 (22.7) |
0.770*
|

Go to :

DISCUSSION
Analyses of data from CHD-VLBWs revealed the following major findings. First, CHD was significantly associated with moderate to severe BPD and severe BPD, and the risk was further augmented by the oxygen supply duration. Second, NEC occurred frequently in CHD-VLBWs and was not associated with their feeding pattern. Third, CHD was associated with increased mortality, where the high mortality can be attributed to cyanotic CHD. Our study had methodological advantages over the before mentioned studies of preterm infants with CHD.
6)16) First, the included data were obtained from a single cohort using a consistent treatment strategy. Second, detailed datasets on the respiratory variables in terms of ventilator use, oxygen supply, and feeding protocol were available. Notably, our results suggest that CHD is a risk factor for rather than simply being associated with BPD, NEC, and mortality in VLBW infants.
The present study confirmed the increased BPD in CHD-VLBWs and the increased duration of the oxygen supply further enhanced the reliability of our results. In preterm infants, BPD is caused by several factors, and postnatal factors include ventilator-induced lung injury and oxygen toxicity.
17) In the case of CHD, ventilator and oxygen use is known to be increased due to impaired cardiac function, pulmonary hypertension, and the open sternum status associated with surgery.
18) Therefore, CHD-VLBWs are generally expected to develop more BPD. Nevertheless, controversy persists regarding the association between CHD and BPD in preterm infants. Pappas et al.
6) reported no relationship between CHD and BPD in extremely low birth weight infants weighing less than 1,000 g but did not perform a further analysis of BPD risk factors such as ventilator duration and oxygen supply. In contrast, Polito et al.
16) reported a 4-fold increase in BPD incidence when CHD coexisted in VLBW infants. However, when the results were further analyzed including ventilator use and oxygen supply, the correlation was weakened.
16) The present study demonstrated that the incidence rates of moderate-to-severe BPD and severe BPD increased in the CHD-VLBWs and were related to oxygen supplementation duration. In the multivariate analysis, cyanotic CHD was related to the incidence of severe BPD in the CHD-VLBWs. In addition, cyanotic CHD-VLBWs more commonly develop BPD than acyanotic CHD-VLBWs, which is consistent with the finding that term infants with cyanotic CHD require longer ventilator support.
19) Norman et al.
20) reported that oxygen supplementation to very preterm infants with CHD when BPD was diagnosed at 36 weeks of postmenstrual age was mainly due to the cyanotic CHD and heart failure that may accompany it. This suggests that unnecessary oxygen supplementation because of concerns about low oxygen saturation can be rather harmful to cyanotic CHD-VLBWs.
CHD was a risk factor that increased the incidence of NEC in VLBW infants. NEC can be caused by intestinal epithelial injury, transmural damage, and perforation of the intestine through inflammation or ischemia.
21) Although the cause of NEC in preterm infants is multifactorial, inflammation is considered the main trigger mechanism versus ischemia.
21) The occurrence of NEC in CHD is known to be due to changes in the hemodynamic circulation.
22) CHD could contribute to bowel ischemia rather than inflammation by decreasing cardiac output and causing cyanosis and congestive heart failure, possibly leading to NEC.
22) In CHD-VLBW infants, in which the 2 risk factors of inflammation and ischemia coexist, the incidence of NEC may be higher. In fact, the incidence of NEC in CHD-VLBW infants was reported 8.6–13.1%
5)23) in previous studies and 11.1% in the present study, which is higher than that of 2–9% in VLBW infants.
21)24) To date, studies indicate that there may be controversy, in that the differences in the incidence of NEC may be caused by the different hemodynamic problems of each CHD.
22) It is logical that bowel ischemia may be caused by the difference in hemodynamic circulation; however, the present study did not identify differences in the incidence of NEC in the analysis of acyanotic versus cyanotic CHD. Early enteral feeding within 96 h after birth may reduce gastrointestinal atrophy, feeding intolerances, and NEC in preterm infants.
13)25) However, the present study suggests that the incidence of NEC may not be related to the feeding pattern, such as the start of feeding and the rate of feeding progression in CHD-VLBWs. For clinicians who are concerned about the feeding protocol due to hemodynamic problems in CHD-VLBWs, the results may be helpful regarding enteral feeding.
It is not surprising that newborns with CHD have a higher mortality rate than those without. This result was consistent with the increase in mortality in the CHD group in this study of VLBW infants. The mortality rate of CHD-VLBWs in this study was 21.5%, similar to the 26.7% reported in a previous study.
6) It is also known that critical CHD, especially cyanotic CHD, has a relatively high mortality rate among all CHD.
4) The majority of cyanotic CHD cases often require surgical treatment in the early days of life; however, when the birth weight is very low, it is challenging to perform surgical treatment. For this reason, cyanotic CHD in VLBW infants is expected to have a higher mortality rate than acyanotic CHD-VLBWs. In addition, this study confirmed that CHD-VLBWs died not only of heart disease but also of prematurity-related diseases. Patterns of causing mortality in CHD-VLBWs were different according to the endurance to the point of cardiac surgery. The major causes of mortality of post-cardiac surgery were complications associated with surgery. On the other hand, if the infant died while waiting for surgery, the major causes of death were related to prematurity itself, probably because the heart problem was not severe enough for the need of urgent cardiac surgery. However, the predominant cause of overall death was surgery-related, so if surgical technique is improved in the future, the mortality rate may be reduced accordingly. In this study, all the patients who underwent PAB died, and PAB was also the leading cause of death after surgery. From previous reports, we can estimate that the success rate is not high even though a few cases of successful PAB in VLBW infants have been reported.
26)27) Therefore, among the various surgical techniques, PAB needs surgical development.
In addition, several studies reported that the prevalence of SGA is 8.6–9.6% in several countries.
28) In this study, the rate of SGA in CHD-VLBWs was 50.6%, higher than other CHD rates of 16.7–27.0%.
6)16) Preterm SGA infants have more morbidities such as BPD, NEC, and LOS and mortalities than infants with appropriate size for gestational age.
29) Therefore, CHD-VLBWs were matched with controls according to gestational age and birth weight to adjust for biases that could influence SGA-related premature morbidities.
This study has some limitations. Above all, the development of neonatology over time was not considered. In the relatively long study period of 10 years, period matching was not performed when matching the control groups. Even during a relatively long study period, the number of CHD-VLBWs was small, so the timing was difficult to match. With the recent advances in neonatology, the incidence of morbidities such as BPD, NEC, IVH, and ROP and the mortality rates of VLBW infants have improved over time. Therefore, if more study subjects are recruited in future studies, the study period must be considered. In the end, the present study was limited by its small population consisting of a retrospective cohort from a single institution. However, since it was limited to a single institution, data of precise ventilator use, oxygen supply, and feeding were available, which provided more accurate information regarding BPD and NEC. In this study, the CHD-VLBWs had increased BPD and NEC, and further studies on strategies for preventing BPD and NEC with a large number of CHD-VLBWs can be expected.
Go to :











 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download