Abstract
Hepatic disorders with prominent cholestasis can be caused by a range of conditions, and anabolic androgenic steroids have been considered a cause of protracted cholestasis. A 29-year-old man who had taken an anabolic androgenic steroid analogue for 2 months visited the hospital complaining of jaundice and indigestion. After stopping the medication, the hyperbilirubinemia tended to decrease, but a transiently elevated aminotransferase level was observed. The endogenous testosterone level also decreased initially but recovered soon after. The liver function profiles were normalized after 2 months of conservative management. This case emphasizes that close drug history taking, including anabolic steroids, is important for identifying the cause of unexplained persistent jaundice.
Go to : 
Jaundice can be caused by a range of conditions; thus, they cannot be identified easily. Primarily, the ratios of the unconjugated and conjugated bilirubin levels may indicate the causes of jaundice.1 Unconjugated hyperbilirubinemia is caused mainly by Gilbert’s syndrome and hemolysis, whereas conjugated hyperbilirubinemia is generally the result of a bile duct obstruction, viral hepatitis, sepsis, and drug-induced liver injury. Most severe jaundice, particularly conjugated hyperbilirubinemia, develops with high liver enzyme levels, including AST, ALT, ALP, and GGT.2 On the other hand, severe jaundice rarely develops without elevated liver enzyme levels. Such patients primarily have genetic disorders, such as Dubin–Johnson and Rotor syndrome, but they seldom have serum bilirubin levels of >10 mg/dL.3 Some patients with toxic or drug-induced liver injury show a similar pattern of jaundice. This paper reports a case of severe jaundice alone in a man who took anabolic androgenic steroid analogues (AAS).
Go to : 
A 29-year-old man visited hospital complaining of icteric sclera and indigestion from a month ago, resulting in inadequate food intake. His body weight had decreased by 6 kg in 2 months. Icteric sclera and yellowish skin were observed 3 days earlier. He had never been diagnosed with any other diseases before, and this was the first time he had jaundice. He had taken AAS, known as selective androgen receptor modulators, to build muscles for 2 months without any other medications. He had no family history of illness. He had not taken any other medicines, such as hepatotonics, before visiting the center. The physical examinations revealed no other abnormalities, such as fever and tenderness, except jaundice.
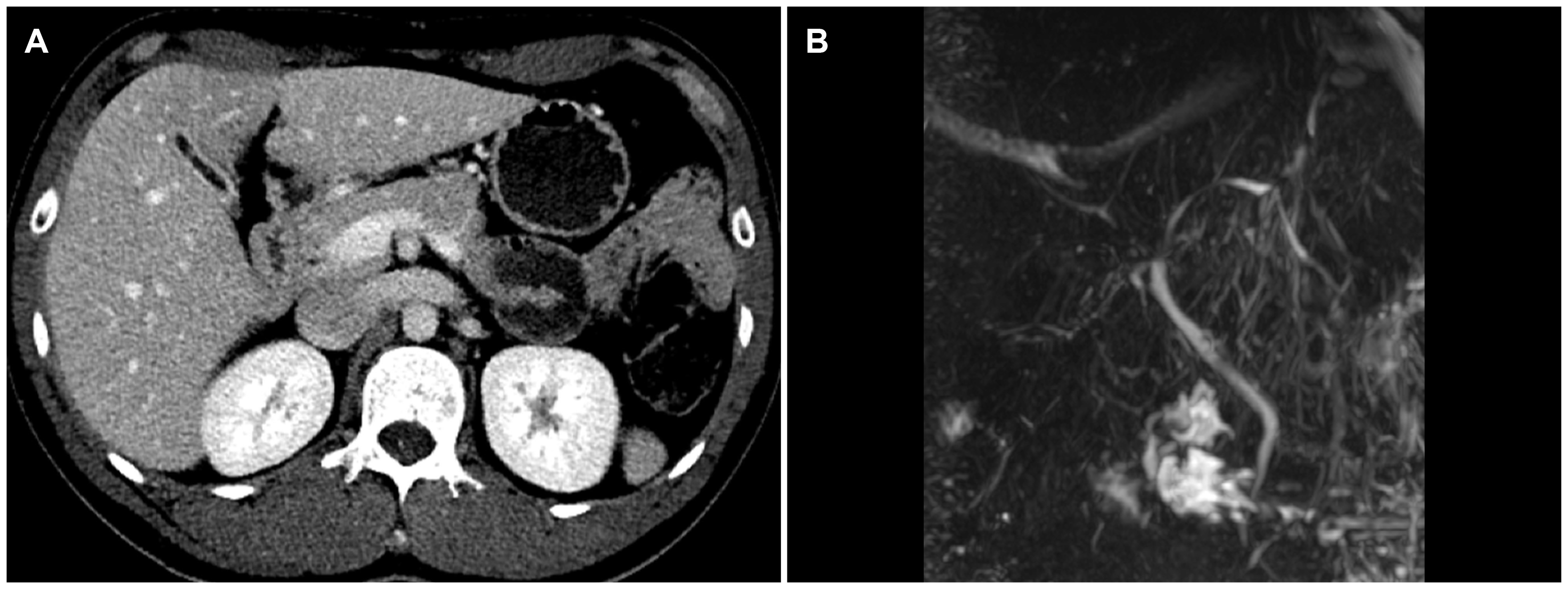
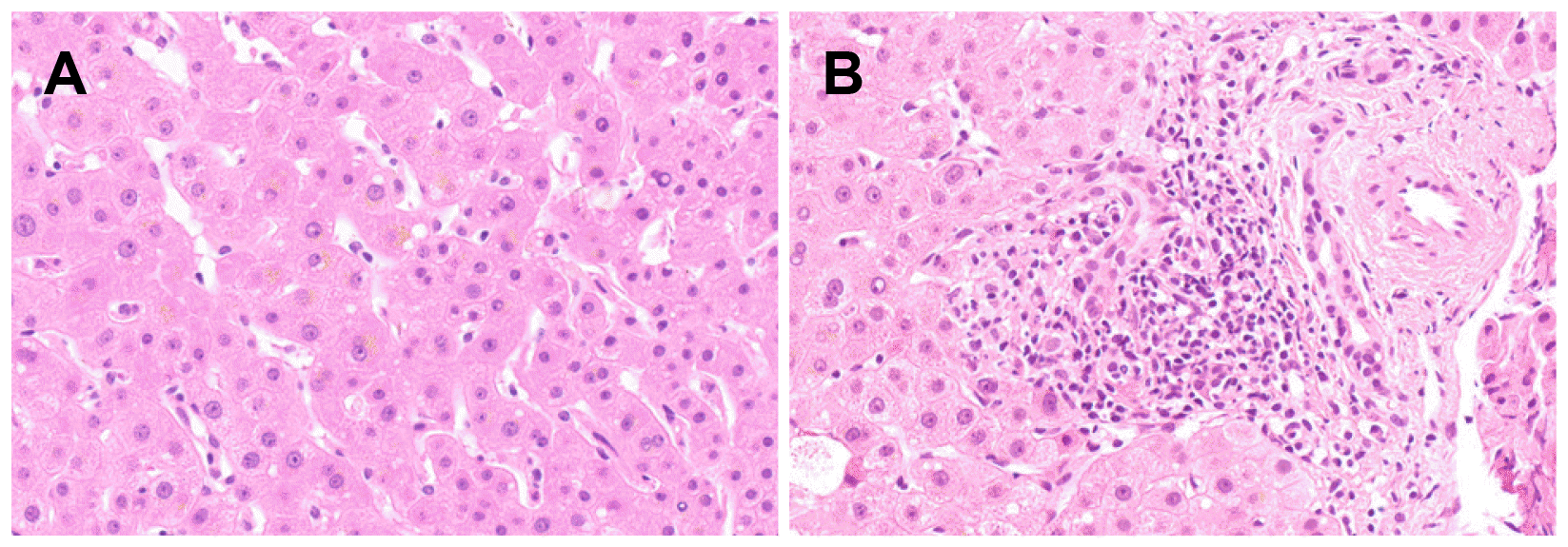
The white blood cell and platelet counts were 4,100 and 288,000/mm3, respectively. The results of the liver function tests were as follows: AST 41 U/L; ALT 80 U/L; ALP 133 U/L; GGT 32 U/L; albumin 4.2 g/dL; total bilirubin 10.11 mg/dL; direct bilirubin 6.25 mg/dL; and PT INR 0.84. He tested negative for the viral markers of hepatitis A, B, C, and E, cytomegalovirus, and Epstein–Barr virus. His tumor markers were within the normal ranges (AFP 2.04 ng/mL; CA 19-9 1.52 U/mL; CEA 1.80 ng/mL). The autoimmune markers, including ANA and AMA, were all negative. Serum IgG, thyroid hormone, and ceruloplasmin levels were also within the normal ranges. The serum bile acid level, however, was higher than 150 (normal range 0-6) μmol/L, and the serum 25-hydroxyvitamin D3 and testosterone levels decreased to 6.77 (normal range 10-30) and 1.68 (normal range 2.5-8.4) ng/mL, respectively. The patient underwent abdominal sonography, CT, and MRI cholangiopancreatography. Except for a collapsed gallbladder, there were no definite abnormal findings, such as biliary tract dilatation and hepatomegaly (Fig. 1). A liver biopsy was performed due to jaundice aggravation, which revealed cholestasis in the hepatocyte cytoplasm and mild lymphocyte and plasma cell infiltrations at the portal tract (Fig. 2).
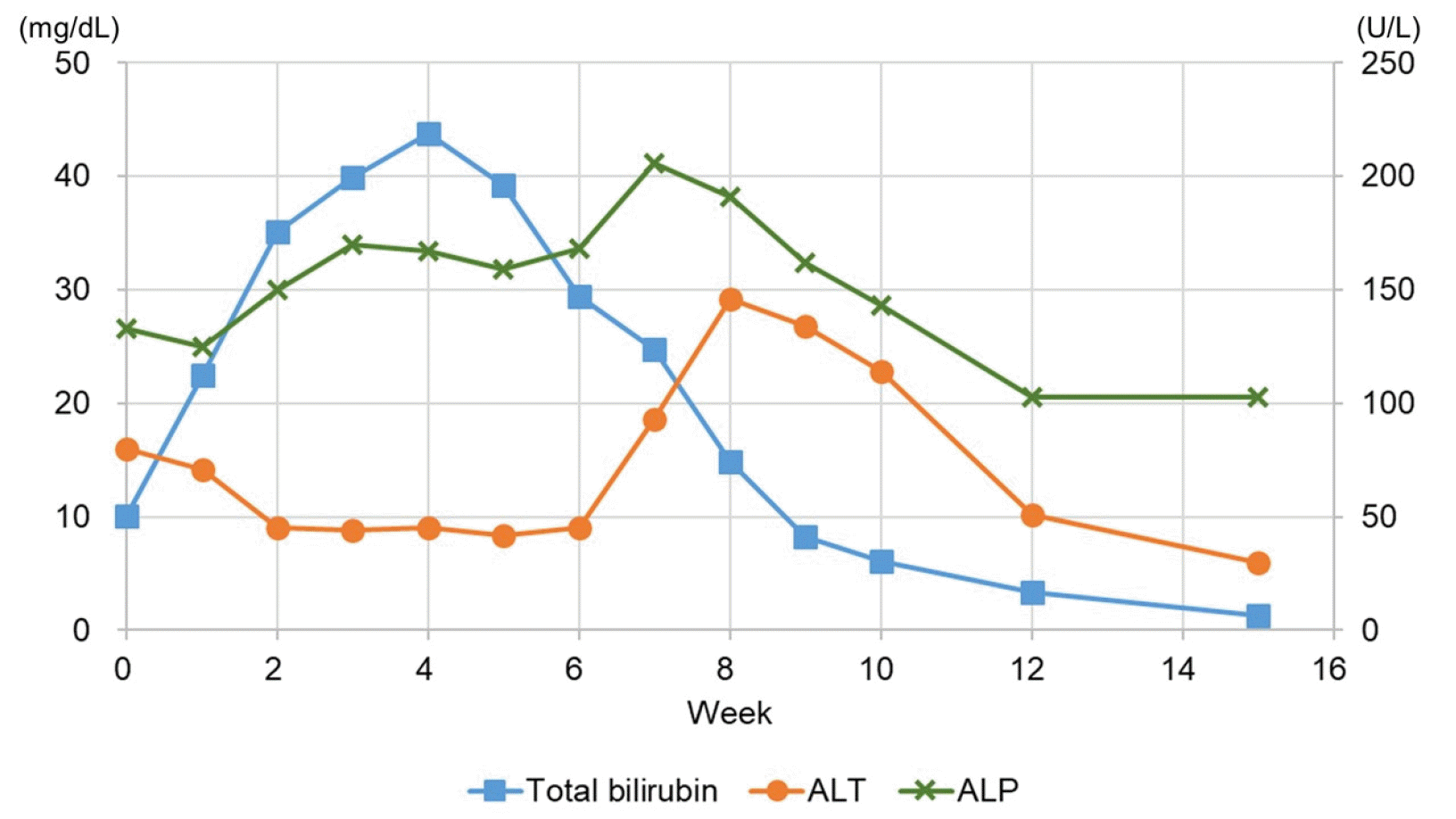
The patient was treated with conservative management, including fluid therapy and ursodeoxycholic acid, and he discontinued AAS. Despite the treatment, his jaundice worsened, and severe pruritus occurred, resulting in an inability to sleep. Antihistamine agents, i.e., mequitazine and hydroxyzine, cholestyramine, naloxone, and sedatives, were added sequentially. One month after the visit, the total and conjugated bilirubin levels peaked at 43.80 mg/dL and 29.35 mg/dL, respectively, and decreased gradually thereafter. Two weeks later, the total bilirubin level decreased to 29.46 mg/dL, and his pruritus improved adequately; thus, he was discharged. Four months after the first visit, the total and direct bilirubin levels returned to 1.3 mg/dL and 0.5 mg/dL, respectively (Fig. 3). The serum bile acid, 25-hydroxyvitamin D3, and testosterone levels also returned to 5.0 μmol/L, 12.70 ng/mL, and 5.54 ng/mL, respectively.
Go to : 
AAS facilitates muscle building in a short time by increasing protein synthesis. They are prescribed clinically for male patients with hypogonadism, breast cancer, aplastic anemia, and hereditary angioneurotic edema.4
On the other hand, the marked efficacy AAS has for muscle building has led to its abuse by performance-obsessed athletes; thus, it has been banned due to social and ethical considerations. Recently, people who enjoy fitness as a hobby have also started abusing this drug for rapid muscle development. These people comprise up to 0.5% of the young population who exercise. Therefore, the number of patients reporting AAS side effects has increased.4,5 AAS intake is associated with a range of side effects, particularly in the liver, with hepatocellular patterns being most common, followed by cholestatic patterns.4 In this case, a young man took an AAS for 2 months, and severe jaundice developed with peak serum bilirubin levels of >30 mg/dL without significantly increasing the liver enzyme levels. The R-value obtained using the ALT/ALP ratio was <2, suggesting the cholestatic pattern of liver injury.6 The Roussel Uclaf Causality Assessment Method (RUCAM) scale score was nine points, suggesting it to be highly probable. The patient stopped taking AAS and started showing positive signs of improvement after 1 month. Although not reported in previous cases, AAS abuse in this patient could be confirmed by the significantly decreased serum testosterone levels, which might be suppressed by AAS.7 In addition, jaundice improved as the testosterone level was normalized, suggesting that the potential effects of AAS abuse will last 2-3 months even after discontinuation. Furthermore, a vitamin D3 deficiency was observed in this case. Previous studies showed that 25-hydroxyvitamin D3 was correlated significantly with the testosterone levels,8,9 and an experimental study proved that the 25-hydroxyvitamin D3 level was decreased significantly during AAS administration.7 Therefore, it could be indirect evidence of AAS abuse, and represents liver injury, which cannot be identified by a typical liver function assessment.10
Previous studies also reported that the serum bilirubin levels increased even after stopping AAS intake and peaked at 30-40 mg/dL. The levels then decreased and completely returned to within the normal limits after 3-5 months.4,11-14 These observations were also consistent with the results in the present case. In the present patient, the transient increase in the ALT level was observed at 8 weeks (Fig. 3), which might be attributed to hormonal dysregulation. As AAS-induced cholestasis began to be resolved, a sudden reactivation of the depressed androgen receptor of hepatic cells might have increased the reactive oxygen species, leading to necro-inflammation.15 In previous cases, severe complications occurred, such as acute kidney injury (AKI) and acute pancreatitis, which required hemodialysis, plasma exchange, or molecular adsorbent recirculating system.4,11,16 One study showed that patients with bilirubinemia (>21.5 mg/dL) were more susceptible to AKI than those without bilirubinemia.4 In the present case, despite the high peak bilirubin levels, AKI did not occur because the patient received sustained fluid therapy during hospitalization.
The precise mechanism underlying the occurrence of cholestasis induced by AAS intake has not been elucidated. An in vitro experiment showed that an AAS metabolite could suppress the activity of the bile salt export pump,17 which is consistent with the high serum bile acid levels upon admission in this case. In addition, cholestasis could be attributed to an intrahepatic canalicular network injury and microfilaments induced by AAS metabolites.15 Therefore, further studies on its mechanism will be needed to determine the appropriate treatment for early recovery from hepatotoxicity. In conclusion, when clinicians encounter patients with unexplained jaundice, detailed medical history taking, particularly the use of anabolic steroids, should be performed. In addition, the public should also be made aware of the exact side effects of AAS.
Go to : 
ACKNOWLEDGEMENT
A waiver of informed consent for this case was approved by the Institutional Review Boards of Korea University Anam Hospital (2020AN0253).
Go to : 
REFERENCES
1. Kwo PY, Cohen SM, Lim JK. 2017; ACG clinical guideline: evaluation of abnormal liver chemistries. Am J Gastroenterol. 112:18–35. DOI: 10.1038/ajg.2016.517. PMID: 27995906.

2. Fargo MV, Grogan SP, Saguil A. 2017; Evaluation of jaundice in adults. Am Fam Physician. 95:164–168. PMID: 28145671.
3. Strassburg CP. 2010; Hyperbilirubinemia syndromes (Gilbert-Meulengracht, Crigler-Najjar, Dubin-Johnson, and Rotor syndrome). Best Pract Res Clin Gastroenterol. 24:555–571. DOI: 10.1016/j.bpg.2010.07.007. PMID: 20955959.

4. Robles-Diaz M, Gonzalez-Jimenez A, Medina-Caliz I, et al. 2015; Distinct phenotype of hepatotoxicity associated with illicit use of anabolic androgenic steroids. Aliment Pharmacol Ther. 41:116–125. DOI: 10.1111/apt.13023. PMID: 25394890.

5. Elsharkawy AM, McPherson S, Masson S, Burt AD, Dawson RT, Hudson M. 2012; Cholestasis secondary to anabolic steroid use in young men. BMJ. 344:e468. DOI: 10.1136/bmj.e468. PMID: 22302781.

6. European Association for the Study of the Liver. 2019; EASL clinical practice guidelines: drug-induced liver injury. J Hepatol. 70:1222–1261. DOI: 10.1016/j.jhep.2019.02.014. PMID: 30926241.
7. Small M, Beastall GH, Semple CG, Cowan RA, Forbes CD. 1984; Alteration of hormone levels in normal males given the anabolic steroid stanozolol. Clin Endocrinol (Oxf). 21:49–55. DOI: 10.1111/j.1365-2265.1984.tb00135.x. PMID: 6430603. PMCID: PMC5605175.

8. Wehr E, Pilz S, Boehm BO, März W, Obermayer-Pietsch B. 2010; Association of vitamin D status with serum androgen levels in men. Clin Endocrinol (Oxf). 73:243–248. DOI: 10.1111/j.1365-2265.2009.03777.x. PMID: 20050857. PMCID: PMC5992301.

9. Lee DM, Tajar A, Pye SR, et al. 2012; Association of hypogonadism with vitamin D status: the European Male Ageing Study. Eur J Endocrinol. 166:77–85. DOI: 10.1530/EJE-11-0743. PMID: 22048968.

10. Kim TH, Yun SG, Choi J, et al. 2020; Differential impact of serum 25-hydroxyvitamin D3 levels on the prognosis of patients with liver cirrhosis according to MELD and Child-Pugh scores. J Korean Med Sci. 35:e129. DOI: 10.3346/jkms.2020.35.e129. PMID: 32419396. PMCID: PMC7234861.

11. Díaz FC, Sáez-González E, Benlloch S, et al. 2016; Albumin dialysis with MARS for the treatment of anabolic steroid-induced cholestasis. Ann Hepatol. 15:939–943. DOI: 10.5604/16652681.1222114. PMID: 27740530.
12. Krishnan PV, Feng ZZ, Gordon SC. 2009; Prolonged intrahepatic cholestasis and renal failure secondary to anabolic androgenic steroid-enriched dietary supplements. J Clin Gastroenterol. 43:672–675. DOI: 10.1097/MCG.0b013e318188be6d. PMID: 19238093.

13. Rosenfeld GA, Chang A, Poulin M, Kwan P, Yoshida E. 2011; Cholestatic jaundice, acute kidney injury and acute pancreatitis secondary to the recreational use of methandrostenolone: a case report. J Med Case Rep. 5:138. DOI: 10.1186/1752-1947-5-138. PMID: 21470406. PMCID: PMC3079674.

14. Niedfeldt MW. 2018; Anabolic steroid effect on the liver. Curr Sports Med Rep. 17:97–102. DOI: 10.1249/JSR.0000000000000467. PMID: 29521706.

15. Solimini R, Rotolo MC, Mastrobattista L, et al. 2017; Hepatotoxicity associated with illicit use of anabolic androgenic steroids in doping. Eur Rev Med Pharmacol Sci. 21(Suppl 1):7–16. PMID: 28379599.
16. El Khoury C, Sabbouh T, Farhat H, Ferzli A. 2017; Severe cholestasis and bile cast nephropathy induced by anabolic steroids successfully treated with plasma exchange. Case Rep Med. 2017:4296474. DOI: 10.1155/2017/4296474. PMID: 29391869. PMCID: PMC5748144.

17. Morgan RE, Trauner M, van Staden CJ, et al. 2010; Interference with bile salt export pump function is a susceptibility factor for human liver injury in drug development. Toxicol Sci. 118:485–500. DOI: 10.1093/toxsci/kfq269. PMID: 20829430.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download