INTRODUCTION
Ulcerative colitis (UC) and Crohn’s disease (CD) are the major entities of inflammatory bowel disease. These disorders are immune-mediated diseases characterized by an imbalance between the regulatory and effector immune responses in the gut where immune cells respond to autoantigens, thereby triggering chronic inflammation.
1 IBD is characterized by the production of pro-inflammatory cytokines, such as interferon (IFN)-γ, tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-17.
2 Current therapies have limitations, despite reducing the production of inflammatory cytokines and influencing the disease outcomes. In addition, patients may become refractory or intolerant and presenting with several side effects.
3 These constrictions reinforce the necessity to develop new therapeutic approaches.
Arthrospira (
Spirulina)
platensis (SPI), commonly known as the nutraceutical spirulina, is a planktonic, filamentous cyanobacterium largely used as a source of bioactive molecules, such as amino acids, vitamins, carotenoids, and minerals. SPI is used frequently for nutrient supplementation in humans without any significant side effects.
4,5 An extract of SPI has also been used to treat inflammatory disturbances in experimental models of arthritis,
6-8 non-alcoholic steatohepatitis,
9 and in patients with allergic rhinitis.
10 The beneficial role of SPI has been addressed in experimental models of 2, 4, 6-trinitrobenzene sulfonic acid (TNBS)
11 and acetic acid-induced intestinal inflammation
12, which have similarities to the pathophysiology observed in CD
13 and UC,
14 respectively. More recently, the beneficial effects of SPI in dextran sulfate sodium (DSS)-induced colitis have been examined in rats.
15
The effects of SPI extracts have never been explored in DSS-induced intestinal inflammation in mice. The DSS model is one of the most used approaches to study inflammatory bowel disease (IBD) and is believed to resemble the features of flares in UC
13 and CD (mainly transmural inflammation).
16 Therefore, this study examined the effects of an SPI extract on the clinical outcomes, colon architecture, and production of the innate and adaptive immune mediators in a murine model of DSS-induced intestinal inflammation.
Go to :

SUBJECTS AND METHODS
1. Cultivation and collection of Arthrospira (Spirulina) platensis
The cultivation of SPI requires intense sunlight, high temperatures and low rainfall, nutrients, and a pH between 9 and 10. Initially, the inocula received from Antenna (Geneve, Switzerland) were cultivated in the authors’ laboratory at the Federal University of Ceara, Brazil, utilizing Zarrouk media. After filtration, the material was weighed to determine the wet biomass and submitted to desiccation for 5 hours (50℃). The dried material was weighed to determine the dried biomass and the production per square meter (8 to 10 g/day/m2).
2. Animal experimentation
C57BL/6 male mice aged 6-8 weeks, weighing 20-25 g, were divided randomly into five groups, with six mice/groups, as follows: healthy-mice not exposed to DSS and treated with saline only; DSS+Vei–mice exposed to 3% DSS and treated with saline by gavage; DSS+50, 100 or 250 mg/kg/day–mice exposed to DSS 3% and treated by gavage with different concentrations of SPI. Colitis was induced by 3% DSS (MP Biomedicals, Illkirch, France, Molecular weight: 36,000-50,000 kDa) added continuously to the drinking water for six consecutive days. Saline and SPI were given orally by gavage for five days. After this period, the mice were euthanized with an intraperitoneal injection of 2, 2, 2-tribromoethanol followed by a cervical dislocation, and the colon samples were collected for immunological and histological assays. The results are representative of two independent experiments. The extract was obtained by macerating SPI dried biomass with saline to obtain the target concentrations.
All animals were housed in specific pathogen-free and standard-controlled environmental conditions at a constant temperature (25℃) on a 12 hours light/dark cycle and provided with food and water
ad libitum in the animal housing facility of the Federal University of Triângulo Mineiro, Brazil. The Institutional Animal Care and Use Committee of Federal University of Triângulo Mineiro approved this study under protocol 388, and all procedures were performed in accordance with the international ethics guidelines. All mice were monitored for their general wellbeing before, during, and after the experiments, as described previously.
17
3. Euthanasia and sample collection
The mice were euthanized on day six, and the colon was removed for further analysis. The colon length was evaluated immediately, and the samples were divided into two smaller sections: 1) immersed into PBS/10% formaldehyde for paraffin embedding; and 2) immersed in a solution containing phosphate-buffered saline (PBS), 2% Nonidet P-40 and protease inhibitors (Complete®, Roche Pharmaceuticals, Mannheim, Germany) for myeloperoxidase (MPO), nitric oxide (NO) and cytokine/chemokine quantification. The dry weights of all sections were determined before storage.
4. Clinical assessment of mice with DSS-induced colitis
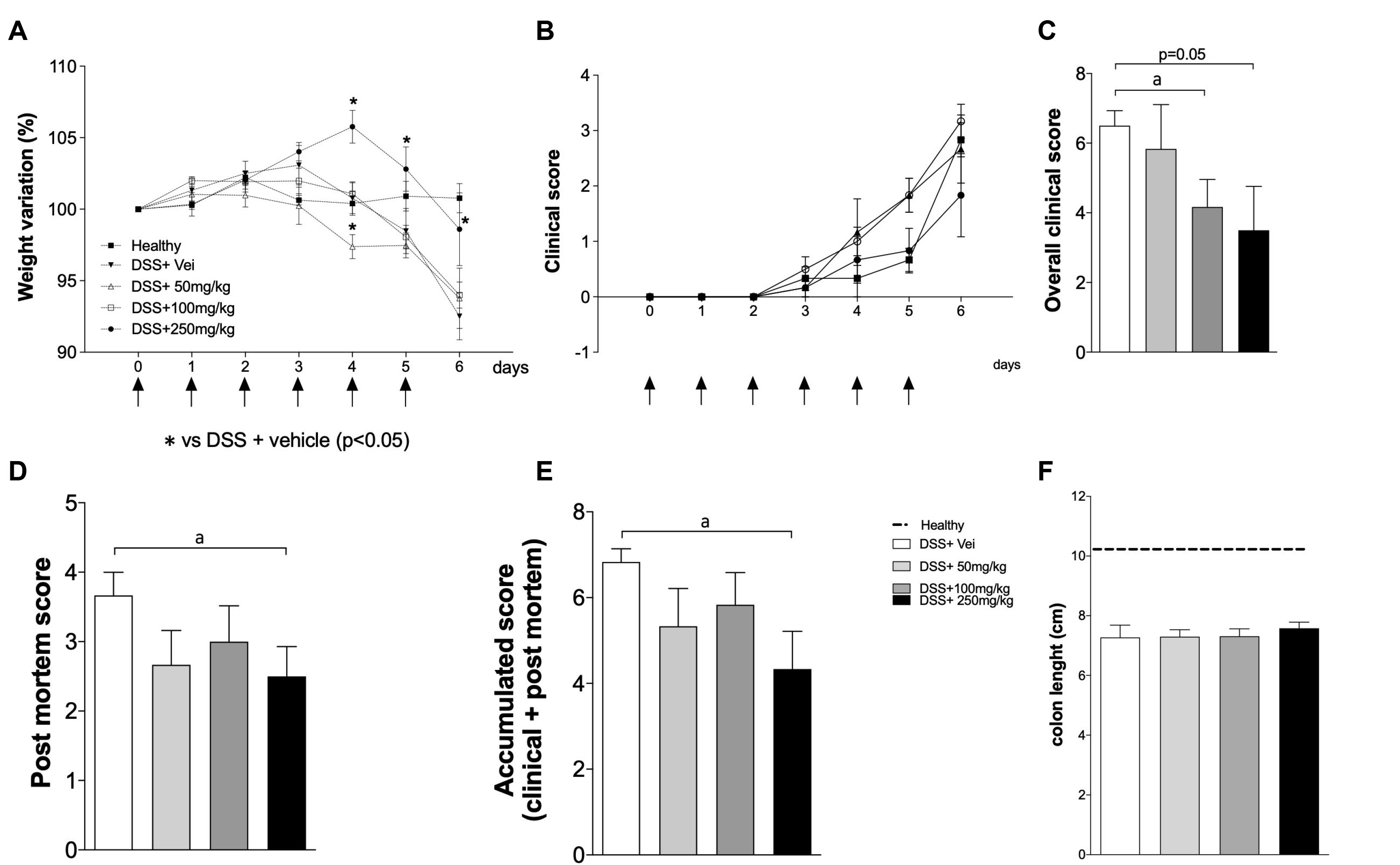
All mice were evaluated daily for their food and water intake, bodyweight variation, and clinical signs of disease. The clinical, overall clinical, post mortem, and accumulated scores were assessed to obtain a disease score for every mouse, as described previously.
18 Briefly, each sign presented by the animals (wet anus, bleeding stools, stools consistency, piloerection, and hypoactivity) corresponded to one point, and the sum of points for each mouse was defined as the overall clinical score. For weight variation, mice that lost 5 to 10% of their body weight in 24 hours received one point. Weight losses higher than 10% received two points. The percentage of weight variation was calculated as follows: the weight of each mouse was determined at the beginning of the study and was considered to be 100%. The weight variation was calculated for each animal during the experimental period based on the daily weight gain or loss and compared to their weight on day 0. The post mortem score was calculated based on the macroscopical alterations of the colon (consistency of stool and bleeding). One point was attributed to each alteration if these alterations were not diffused. Regarding the diffused alterations, two points were attributed to each alteration observed. The accumulated score was calculated based on the clinical score on the day of euthanasia and the macroscopic score. The sum of the signs or macroscopic disturbances for each mouse was represented graphically as a bar chart.
5. Intestinal homogenate preparation
Colon sections stored in PBS containing the protease inhibitors were kept at 4℃ and disrupted mechanically using TissueRuptor II (Qiagen, Germantown, MD, USA) until visual homogenization was achieved. The samples were then centrifuged at 1,000 xg, and the supernatants were stored at -80℃ until the MPO, NO, and cytokine quantification.
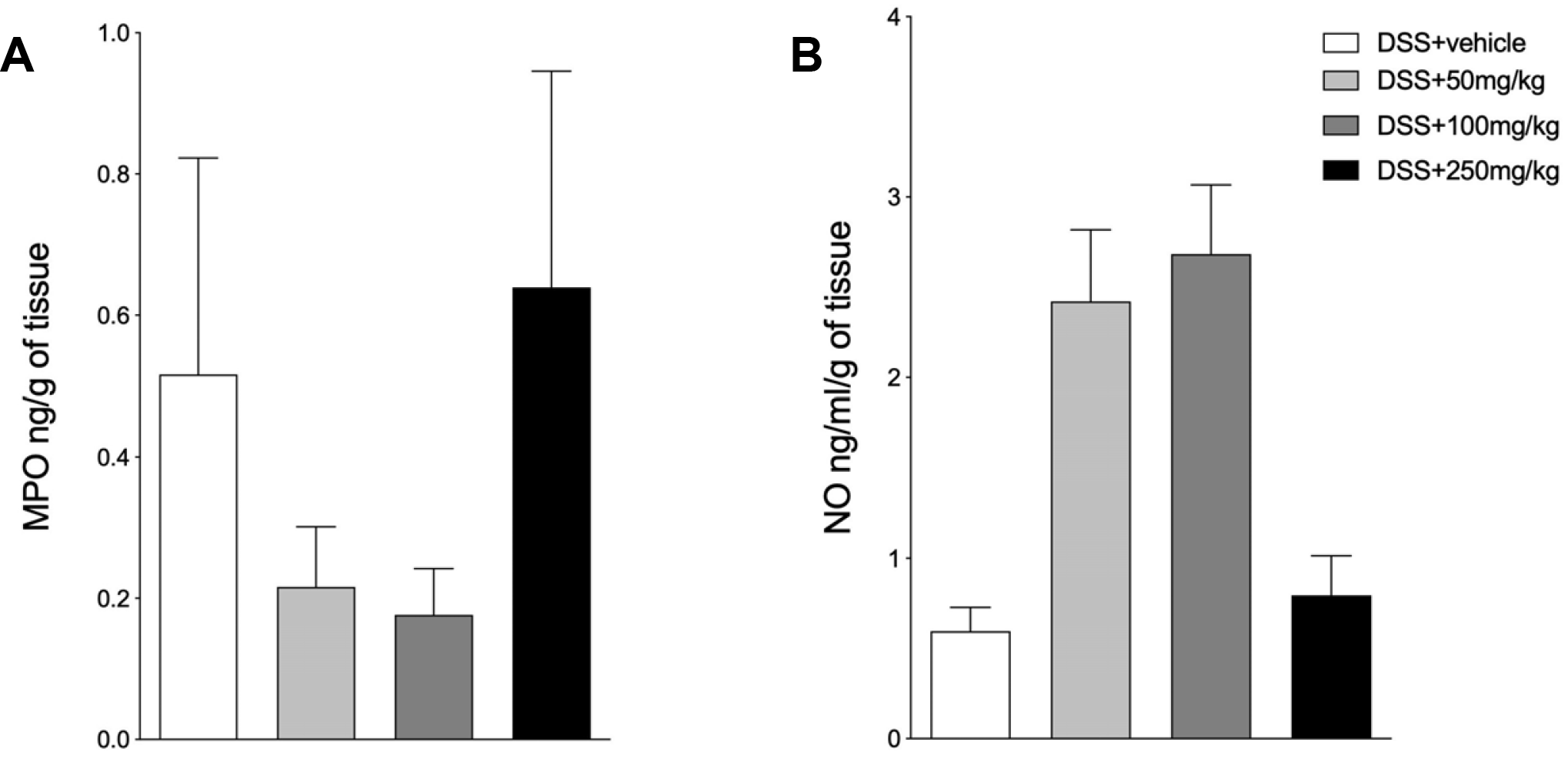
6. MPO and NO analysis
The activity of MPO was determined as previously described.
19 Briefly, a tetramethylbenzidine substrate (BD OptEIA™, San Diego, CA, USA) was added to colon homogenate samples in 96-well plates at 37℃. The reaction was stopped, and readings were performed in a spectrophotometer (Perkin Elmer Cetus, CA, USA) at 450 nm. The results were normalized to the dry weight of each colon section and are expressed as the optical density per gram of tissue (nm/g of tissue).
For the nitric oxide measurements, the nitrite accumulated in the colon homogenates was measured as an indicator of NO production using a Griess reaction.
20 The tissue homogenate was mixed 1:1 with Griess reagent (1% sulfanilamide in 5% phosphoric acid and 0.1% naphthyl-ethylenediamine-hydrogen chloride) and incubated at room temperature for 10 min. The absorbance was measured at 540 nm in a 96-well plate reader (Perkin Elmer Cetus). The amount of nitrite in the samples was calculated based on the absorbance of a serial dilution of the sodium nitrite standard curve. The results were normalized to the dry weight of each colon section and are expressed as picograms per milliliter per gram of tissue (pg/mL/g).
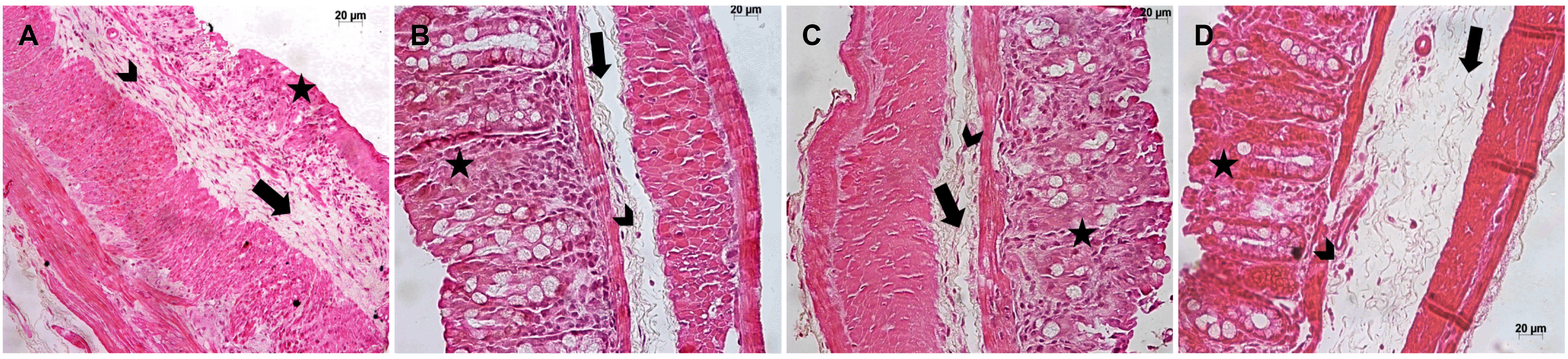
7. Histopathological analysis
To assess the microscopic damage induced by DSS in mice treated with or without SPI, the colon sections were cut longitudinally, washed with PBS, fixed in 10% buffered formalin for 24 hours, and then processed for paraffin embedding followed by microtome sectioning. Tissue sections (5 μm) were obtained and stained with hematoxylin and eosin. For histopathological analysis, the mucosa, submucosa, muscle layers, and serosa were evaluated. These colon sections were also assessed for the presence of edema, inflammatory infiltrate, and epithelial abnormalities.
Images were captured using a digital video camera (Axion camicC5, Zeiss, Berlin, Germany) coupled to an optical microscope (Nikon Eclipse 50i, Nikon, Melville, NY, USA). Semi-quantitative analyses of infiltrating edema and epithelial abnormalities were also performed and classified according to the level of tissue involvement as follows: mild (>25%), moderate (25-50%), or severe (>50%). A trained pathologist who was blinded to the treatment performed the histopathological analysis.
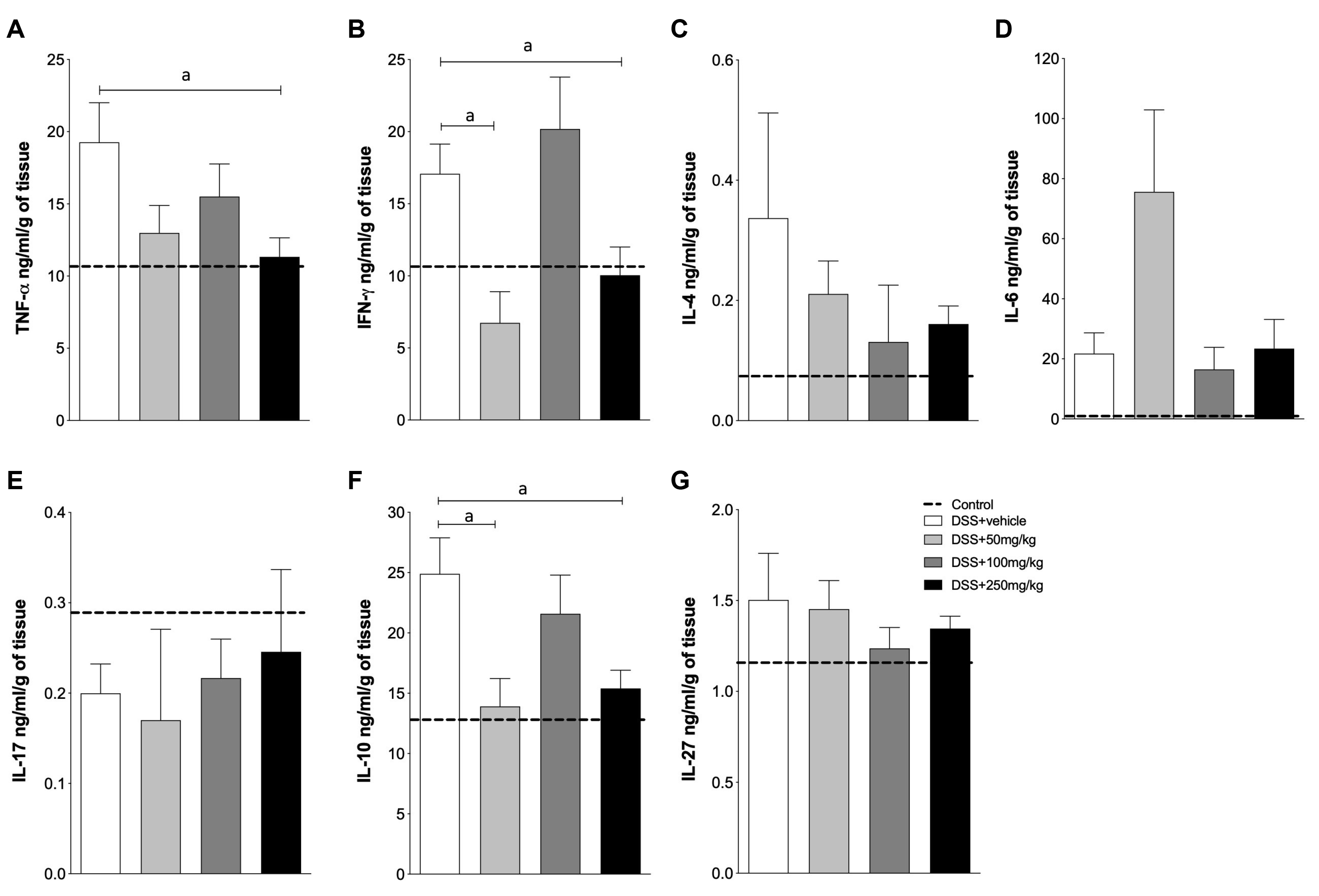
8. Cytokine quantification by ELISA
Cytokines IFN-γ, TNF-α, IL-4, IL-6, IL-10, IL-17 (BD Biosciences, San Jose, CA, USA) and IL-27 (Invitrogen eBioscience, Vienna, Austria) were quantified in the colon homogenates by ELISA according to the manufacturer’s instructions. The results were normalized to the dry weight of each intestinal section and are expressed as nanograms per milliliter per gram of tissue (ng/mL/g of tissue).
9. Statistics
The number of animals per group (n:6) was estimated based on previous publications from the authors’ group using C57BL/6 mice exposed to DSS. The sample size was determined by assessing the feasibility of minimally detectable effect sizes. All results are representative of two independent experiments. The normal distribution and homogeneous variance were tested for all the variables. Parametric tests were used when the distribution was considered normal, and the variance was homogeneous: unpaired Student’s t-test or one-way analysis of variance followed by Tukey’s post hoc test. The following nonparametric tests were used in cases of non-Gaussian distribution of data: Mann-Whitney test or Kruskal-Wallis test accompanied by a Dunn’s post hoc test. The results are expressed as mean±SD. The differences observed were considered significant when p<0.05. Statistical analysis was performed using GraphPad Prism, version 7.0 (GraphPad Software, La Jolla, CA, USA).
Go to :

DISCUSSION
This study examined the anti-inflammatory properties of an extract of SPI in DSS-induced intestinal inflammation. At the highest dose, the extract could ameliorate the clinical outcomes and intestinal architecture as well as reduce the local production of inflammatory cytokines in the intestine, which are related to disease worsening and are used as a target for other therapeutic approaches.
The exacerbated immune response in IBD leads to the production of inflammatory cytokines, such as IFN-γ, IL-12, IL-6, TNF-α, IL-17, and IL-23, which contribute to the development and maintenance of chronic inflammation.
21,22 The current therapies are aimed at modulating the inflammatory mediators, inducing remission, and reducing disease relapse. Nevertheless, even the immune biologics targeting cytokines present several side effects and may induce intolerance.
3,22 This scenario highlights the need to develop therapeutic strategies with greater efficacy.
The anti-inflammatory activity of SPI appears to be related to the inhibition of NO, prostaglandin E
2 (PGE
2), the transcription factor NF-κB, cyclooxygenase-2 (COX-2), TNF-α, and IL-1β, as described previously.
23,24 These properties can be at least partly attributed to phycocyanin. Phycocyanin is one of the main molecules found in spirulina that has significant antioxidant and anti-inflammatory activities. The compound can suppress the expression and production of inducible nitric oxide synthase, nitrites, as well as inhibit liver microsomal lipid peroxidation and the expression of COX-2 and PGE
2.
23 Although this study did not examine if the effects observed in the present study were due to the activity of phycocyanin, the role of phycocyanin in immune modulation and clinical amelioration in DSS-induced colitis cannot be underestimated.
Spirulina has been used to re-establish the immune balance in different scenarios owing to its anti-inflammatory and immunomodulatory properties. Treatment with SPI at 200 or 400 mg/kg inhibited the development of macroscopic and microscopic lesions in Freund’s adjuvant-induced arthritis in rats and improved the clinical outcomes compared to the untreated controls.
8 Furthermore, SPI decreased the levels of COX-2, TNF-α, and IL-6 in the serum.
8 Similarly, using
S. maxima, the anti-inflammatory effects of
Spirulina spp were reinforced in an experimental model of chronic inflammation induced by Freund’s complete adjuvant in rats.
25 The therapeutic and anti-inflammatory properties of SPI were also explored in experimental models of IBD, such as 2, 4, 6-TNBS, acetic acid-induced inflammation, and DSS. The oral administration of 2 g/kg of SPI in Wistar rats 7 days after TNBS exposure induced a reduction in inflammation on the mucosa and submucosa layers, along with a decrease in necrosis, cellular infiltration, and crypt abscess formation compared to the untreated group.
11 The administration of SPI at 500 mg/kg to rats in an acetic-acid model of intestinal inflammation could improve the clinical score and reduce the production of MPO, PGE
2, TNF-α, IL-1β, and IL-6 in the intestine.
12 The oral administration of the hydroalcoholic extract of SPI at 200 mg/kg also promoted disease amelioration and reduced the DSS-related inflammatory changes, including a reduction in TNF-α and IL-6.
15
Interestingly, mice treated with the lowest (50 mg/kg) or the highest (250 mg/kg) doses showed lower levels of the anti-inflammatory cytokine IL-10 compared to those with intestinal inflammation receiving the vehicle only. At the highest dose, this suggests that the modulation of inflammation relied more on the reduction of the key inflammatory cytokines, such as IFN-γ and TNF-α, than on the induction of anti-inflammatory molecules, such as IL-10. The better clinical outcome and colon architecture observed at the highest dose corroborate this hypothesis. Despite the differences in the experimental models of inflammation, SPI concentrations, nature of the extract, and duration of treatment used in previous studies, SPI appears to be an important modulator of inflammation in intestinal inflammation. These results are in accordance with previous publications concerning the downregulation of inflammatory cytokines, particularly TNF-α and IFN-γ.
Furthermore, SPI could protect and reduce intestinal damage in mice challenged with DSS. Although the effects of SPI on the production of the inflammatory mediators, PGE
2 and COX-2, were not evaluated, the contribution of such modulation to constrain inflammation and disease worsening cannot be excluded. In addition, treatment with SPI had no significant impact on the modulation of MPO. Intriguingly, despite the lack of statistical significance, the doses of 50 and 100 mg/kg tended to induce higher levels of NO production, which indicate a dose-dependent paradoxical effect of the SPI extract. In DSS-induced inflammation in rats and using a hydroalcoholic extract, however, treatment with SPI at 100 and 200 mg/kg reduced the production of MPO.
15 These differences could, at least in part, be attributed to the treatment duration and the different natures of the extracts used in the studies (saline vs. hydroalcoholic), which may have resulted in different molecular compositions.
Despite the beneficial effects observed when the SPI extract was given to the DSS-exposed mice, particularly when the highest dose was used, this study had some limitations that need to be considered when interpreting these findings. First, the molecule in the SPI extract responsible for the observed effects was not identified. Second, a dose-dependent effect was not observed in some assays, such as the determination of NO, MPO, and some cytokines. The crude extract, which is a complex formed by molecules with distinct chemical and biological characteristics and in different amounts, is not homogenous, which may have contributed to this observation. Even isolated molecules, such as PGE
2, can present either pro- or anti-inflammatory properties, depending on the cell signalization, context, and dosage.
26,27 This observation suggests the presence of molecules in the saline extract with putative ambiguous activity, acting as pro- or anti-inflammatory mediators depending on the dose, but such properties need to be explored further. Third, the nature of the extract, which was obtained by macerating crude SPI with saline, may also have contributed to the isolation of compounds with different chemical and biological properties.
In conclusion, these findings suggest an immunomodulatory property of SPI in modulating IFN-γ and TNF-α, which are the key cytokines in the development of inflammatory and autoimmune diseases, such as IBD. Furthermore, SPI, particularly at 250 mg/kg, attenuated DSS-induced intestinal architecture disturbances and positively affected the clinical outcomes.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download