INTRODUCTION
Standard triple therapy, containing a proton pump inhibitor (PPI), amoxicillin, and clarithromycin, is one of the most popular regimens as a first-line eradication therapy for a
H. pylori infection. On the other hand, the efficacy of standard triple therapy is decreasing worldwide.
1 This trend has also been noticed in Korea, and the reported eradication rates of
H. pylori using standard triple therapy were 84.9-87.5% from 2001 to 2007 and 80.0-81.4% from 2008 to 2010 (p<0.0001), showing a declining tendency over the past 10 years in Korea.
2 Therefore, a potent first-line regimen is required for successful
H. pylori eradication.
Several components influence successful eradication, including antibiotic resistance of
H. pylori, compliance of the patients, and host and bacterial factors. Acid suppression is also a critical factor in eradication. Thus, the possibility that the type of PPI could affect the eradication rate was suggested. Among the PPIs, esomeprazole, which is the S-isomer of omeprazole, was the first PPI available for clinical use as an optical isomer.
3 Esomeprazole is less metabolized by cytochrome P450 2C19 (CYP2C19) than omeprazole. Therefore, it has a higher bio-availability and greater gastric acid suppression than omeprazole.
3,4 The metabolic advantage of esomeprazole is that it enhances the plasma concentration, leading to a higher area under the curve.
5
Recent studies using potassium-competitive acid blockers (P-CABs) reported a rapid onset of action and dose-dependent effects on acid production, but P-CABs are not available outside Asia, Europe, and the United States.
6 Therefore, this study compared the efficacy of a seven-day high-dose esomeprazole-based triple therapy as a first-line
H. pylori eradication treatment with a seven-day standard dose non-esomeprazole-based triple therapy. In addition, the risk factors associated with eradication failure were identified.
Go to :

SUBJECTS AND METHODS
1. Study population
This retrospective study included patients who visited Kosin University Gospel Hospital from June 2016 and January 2017 and were diagnosed with a H. pylori infection, for which they received first-line PPI-containing triple therapy. H. pylori positivity was verified by a rapid urease test or a 13C-urea breath test before and after the eradication therapy. Compliance was classified as good or poor by the pill count based on the medical records. Participants who consumed ≥80% of the prescribed medicine were entered into the good compliance group, and those who consumed <80% of the prescribed medicine were placed in the poor compliance group. Patients were excluded if they were poorly compliant or lost to follow-up. They were also excluded if they underwent 10-14 days of first-line PPI-containing triple therapy or another antibiotic-based eradication therapy, including metronidazole.
With regard to the demographic features, this study evaluated the alcohol and smoking habits, comorbidities (e.g., diabetes mellitus or hypertension), area of residence, endoscopic diagnoses, and side effects of eradication therapy. Urban residence was factored if the participants lived in metropolitan cities of Korea, and rural residence was considered if the participants did not reside in metropolitan cities. Endoscopic findings, such as gastric ulcers, duodenal ulcers, gastric and duodenal ulcers, previous endoscopic submucosal dissection state due to adenoma or early gastric cancer, mucosa-associated lymphoid tissue lymphoma, gastritis, dyspepsia, and gastric polyps were verified by gastroscopy or gastroscopy with a biopsy. The adverse effects of eradication therapy were confirmed by reviewing the medical records.
2. H. pylori eradication therapy and follow-up
The seven-day high-dose esomeprazole-based triple therapy or seven-day standard dose non-esomeprazole-based triple therapy was prescribed for patients undergoing first-line H. pylori eradication therapy. High-dose esomeprazole-based triple therapy consisted of 40 mg of esomeprazole, 1 g of amoxicillin, and 0.5 g of clarithromycin twice daily for seven days (7-HEAC). The standard dose non-esomeprazole-based triple therapy included a standard dose of PPI except esomeprazole, 1 g amoxicillin, and 0.5 g clarithromycin twice daily for seven days (7-NEAC). The standard dose non-esomeprazole PPIs was comprised of rabeprazole 20 mg and lansoprazole 30 mg.
Subsequently, a 13C-urea breath test or a rapid urease test was performed to assess the extent of H. pylori eradication at least 4 weeks after completing the treatment. Before the 13C-urea breath test or a rapid urease test was performed, the patients discontinued PPI or histamine (H2) receptor antagonist treatment for at least 2 weeks.
3. Rapid urease test
Endoscopic biopsy of the gastric mucosa was conducted to identify a H. pylori infection with the rapid urease test (CLO test®, Delta West, Bentley, Australia). The antrum and corpus were the sites of the gastric mucosal biopsy, and normal or near-normal gastric mucosa with minimal atrophy or intestinal metaplasia was acquired. The tissue sample was immersed in the rapid urea reagent. The result was deemed positive when the reagent color changed from yellow to red at least 12 hours later. The result was deemed negative if there was no change in reagent color.
4. 13C-urea breath test
The patients fasted for at least 4 hours before collecting the first breath sample. The patients then consumed tablets containing 100 mg of 13C-urea (UBiTkitTM, Otsuka Pharmaceutical, Tokyo, Japan) with 100 mL of water orally. The second breath sample was collected 20 min after taking the tablets. The breath samples obtained were analyzed using a 13C-urea breath test (UBiT-IR300®; Otsuka Electronics, Osaka, Japan). The cut-off value of the procedure was set to 2.5‰.
5. Statistical analyses
All statistical analyses were conducted using the Statistical Package for the Social Sciences software version 20.0 (SPSS, Chicago, IL, USA). The
H. pylori eradication rate was evaluated through per-protocol (PP) analysis. The categorical variables and continuous variables were analyzed using a Chi-square (χ
2) test and a Student’s
t-test, respectively. The results of univariate and multivariate logistic regression analyses of the risk factors were expressed as the odds ratios (ORs) and 95% CI. p-values<0.05 were considered significant.
Go to :

DISCUSSION
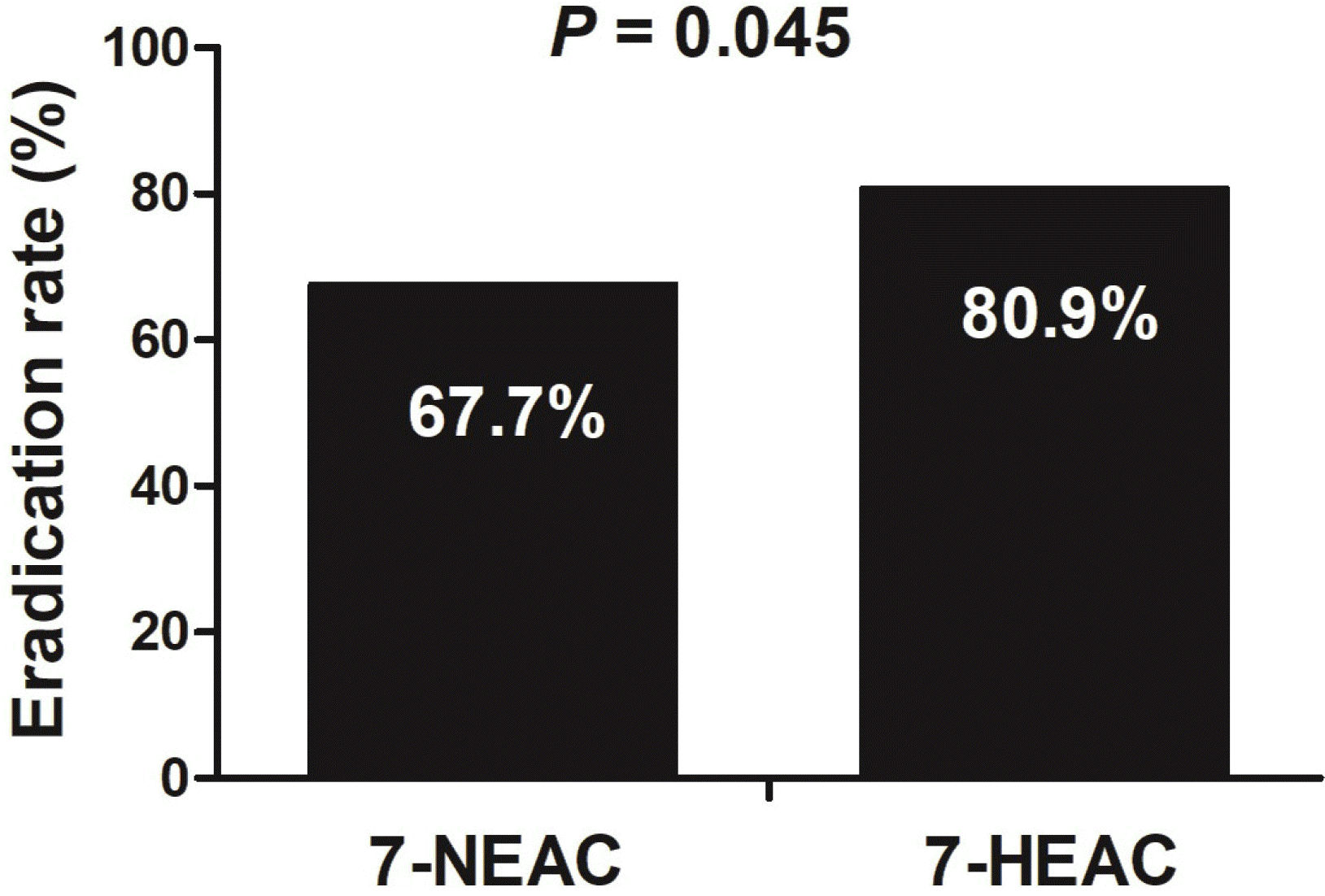
In the current study, the H. pylori eradication rates associated with the 7-NEAC and 7-HEAC regimens were 67.7% and 80.9%, respectively, and adverse events were noted in 5.8% of the 7-NEAC group and 7.4% of the 7-HEAC group. Therefore, the 7-HEAC group had a significantly higher eradication rate than the 7-NEAC group, and the adverse effects of 7-HEAC were similar to those of 7-NEAC. In addition, being female contributed to eradication failure.
These results are in agreement with previous studies comparing the
H. pylori eradication rates associated with high-dose PPI-based triple therapy and standard-dose PPI-based triple therapy. A high-dose of PPI refers to a double-dose of PPI.
7 A prospective Greek study showed that the eradication rate of high-dose (40 mg b.i.d.) esomeprazole-based triple therapy was significantly higher than that of the standard dose (20 mg b.i.d.) omeprazole-based triple therapy.
8 In particular, the eradication rate was 96% (95% CI 91-99%) in the high-dose esomeprazole-based triple therapy group.
8 Another study from Taiwan reported that the eradication rate of esomeprazole-containing triple therapy (40 mg b.i.d.) was significantly higher than that of pantoprazole-containing triple therapy (40 mg b.i.d.).
9 Meta-analysis also evaluated the benefits of high-dose PPI in seven-day standard triple therapy.
10 A total of 1,703 patients from the six studies were included, and a mean intention-to-treat (ITT) eradication rate of 82% was found in the high-dose PPI group compared to the 74% in the standard dose PPI group (risk ratio 1.09; 95% CI 1.01-1.07).
10 Therefore, the meta-analysis showed that high-dose PPI was more efficient than standard-dose PPI in standard triple therapy.
Some studies reported different results from the present study. Eradication rates of triple therapy based on four different PPIs (omeprazole 20 mg b.i.d., pantoprazole 40 mg b.i.d., rabeprazole 20 mg b.i.d., and esomeprazole 40 mg b.i.d.) were similar in a Korean study (p=0.517).
7 The eradication rates in the omeprazole, pantoprazole, rabeprazole, and esomeprazole groups were 64.9%, 69.3%, 69.3%, and 72.9%, respectively.
7 A recent Italian study reported that according to ITT and PP analyses, there were no differences in the eradication rate between standard dose PPI-containing triple therapy (esomeprazole 20 mg b.i.d.) and high-dose PPI-containing triple therapy (esomeprazole 40 mg b.i.d.) groups (ITT analysis, 73.9% vs. 81.9%, p=0.25; PP analysis, 78.2% vs. 85.5%, p=0.27).
11 Hsu et al.
4 reported no significant difference in the
H. pylori eradication rates between high-dose esomeprazole-containing triple therapy (40 mg b.i.d.) and low-dose esomeprazole-containing triple therapy (40 mg q.d.) (p>0.05).
Several studies comparing high-dose PPI-based and standard-dose PPI-based triple therapy showed that the eradication rates differed depending on the type of PPI. In the majority of studies, there were no significant differences in the eradication rates with esomeprazole 40 mg twice daily and esomeprazole 20 mg twice daily or 40 mg once daily.
4,11,12 The cytochrome P450 system determines the metabolism of PPIs.
5 Among them, PPIs are catalyzed mainly by CYP2C19 and cytochrome P450 3A4 (CYP3A4). Therefore, a CYP2C19 polymorphism could be a major factor in the treatment of PPIs.
13 The degree of PPIs metabolism via CYP2C19 varies according to the types of PPIs. Omeprazole, lansoprazole, and pantoprazole are metabolized extensively by CYP2C19.
13 In contrast, rabeprazole is metabolized prominently through a non-enzymatic pathway with a minor CYP2C19 association. Thus, rabeprazole is less affected by the CYP2C19 genotype and has little interaction with other drugs.
13 As mentioned above, esomeprazole has higher bio-availability and greater acid suppression effect in the stomach.
3,4 In addition, esomeprazole therapy leads to fewer inter-individual variations in acid control and a more rapid onset of acid suppression.
4,14 Therefore, esomeprazole is an effective and powerful acid inhibitor compared to other conventional PPIs, which affect the eradication rates by the persistent anti-
H. pylori effect.
The role of acid suppression in eradication therapy is based on the following: 1) the direct antibacterial activity of PPI, 2) inhibition of urease activity, and 3) enhanced activity and stability of antibiotics.
15 Among them, increased activity and antibiotic stability via acid suppression are the most notable because they are associated with the
H. pylori bioenergetics.
15 According to transcriptomics studies, the genes underlying
H. pylori cell division and cell wall synthesis showed elevated transcription at neutral pH compared to acidic pH (pH 4.5).
16 Because the antibiotic effects of amoxicillin and clarithromycin are determined by bacterial growth and cell envelope synthesis, their bactericidal effect is activated in dividing bacteria.
16 Sugimoto et al.
17 used 24 hours intragastric pH monitoring to determine if gastric acid suppression affected eradication therapy. The median 24 hours gastric pH values were 6.4 and 5.2 in patients with and without successful eradication, respectively (p=0.0131), and successful eradication rates were associated significantly with the degree of acid suppression.
17
The eradication rates of the study were still unsatisfactory. An eradication rate higher than 90% is considered as the optimal cut-off therapy for PP analysis.
18 On the other hand, a meta-analysis showed that the overall eradication rate of standard triple therapy from 1998 to 2013 was 82.0% (95% CI 80.8-83.2%) based on PP analysis in 104 studies with 42,124 Korean patients.
19 Between 2017 and 2018, a nationwide prospective multicenter study was conducted in Korea to evaluate the antibiotic resistance rates of
H. pylori. Overall, 580 patients were enrolled, and the resistance rates against clarithromycin, metronidazole, levofloxacin, ciprofloxacin, amoxicillin, and tetracycline were 17.8%, 29.5%, 37.0%, 37.0%, 9.5%, and 0%, respectively.
20 A recent Korean study conducted in the same region as the current study reported antibiotic resistance rates of clarithromycin, metronidazole, levofloxacin, ciprofloxacin, amoxicillin, and tetracycline of 19.3%, 19.3%, 40.9%, 40.9%, 11.3%, and 0%, respectively.
21 Therefore, more effective first-line eradication therapies, including intensive acid suppression, are required in countries showing increased antibiotic resistance, such as Korea.
Being female (OR 2.08; 95% CI 1.15-3.76) was associated with
H. pylori eradication failure in this study. Several studies reported that female gender influenced
H. pylori eradication.
22-25 Moayyedi et al
.26 speculated that the gastric physiology probably differed between males and females. A Korean study that investigated clarithromycin mutation rates using the point mutation of the 23S rRNA gene reported that females were infected predominantly with clarithromycin-resistant
H. pylori carrying the A2143G mutation (p<0.005).
27 Therefore, females with this point mutation in the 23S rRNA of
H. pylori strains contributed to the failure of PPI-containing triple therapy, including clarithromycin. Another Korean study, however, found no significant difference in clarithromycin-resistant
H. pylori between males and females based on point mutations in the 23S rRNA gene (p=0.087).
25 Therefore, gender differences in the eradication of
H. pylori need to be evaluated in the future.
The study limitations relate to the lack of a histological diagnosis of
H. pylori before and after eradication therapy. A few patients underwent both a rapid urease test and a
13C-urea breath test for the confirmation of
H. pylori. These limitations influence the eradication rate. On the other hand, compared to the 80% sensitivity of
H. pylori based on histology,
28 the sensitivity and specificity of the rapid urease test ranged from 90% to 95% and 95% to 100%, respectively.
29 Furthermore, the sensitivity and specificity of the
13C-urea breath test were 95% to 97% and 91% to 94%, respectively.
30,31 The accuracy of both tests is high and very convenient for clinical use.
32 Thus, the absence of histology is unlikely to have affected the study significantly.
In conclusion, treatment with high doses of an esomeprazole-containing triple-drug combination for seven days leads to a higher eradication rate than the standard dose non-esomeprazole-containing triple-drug regimen for seven days, and the adverse effects of both regimens are acceptable. Being female is associated with a higher risk of eradication failure. On the other hand, low eradication rates of H. pylori are still under consideration. Therefore, further well-designed and large-scale studies examining the efficacy of first-line eradication therapies for H. pylori are required in Korea, including studies with potent acid suppression.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download