Abstract
Eosinophilic esophagitis (EoE) is an immune or antigen-mediated chronic inflammatory esophageal disorder that is relatively rare in Asian countries. The main symptoms of EoE are dysphagia and food impaction. Although chest pain is a symptom of EoE, it is also a symptom of coronary heart disease. This paper reports a case of EoE with angina pectoris in a 45-year-old male who was referred to the authors’ hospital for chest pain. He was diagnosed with angina pectoris because of mild stenosis in the left coronary artery on coronary angiography. On the other hand, the symptoms did not improve with angina medication therapy. Therefore, he underwent a chest CT scan, which revealed esophageal thickening. Esophagogastroduodenoscopy was performed. His endoscopic findings showed linear furrows with edema, and >90 eosinophils existed per high-power field on the histology findings. He was diagnosed with EoE. Through additional examinations, he was also diagnosed with asthma. The patient was treated with a proton pump inhibitor and a fluticasone inhaler. His symptoms and abnormal endoscopic findings disappeared after eight weeks of treatment. This case shows that physicians should consider the possibility of the symptoms for EoE when unexplained chest pain persists.
Go to : 
Eosinophilic esophagitis (EoE) is a chronic, immune, or antigen-mediated esophageal disorder associated with the symptoms related to esophageal dysfunction and eosinophil-predominant inflammation.1 The incidence of EoE has increased in Western countries in recent years but is relatively rare in Asian countries, and few studies have been conducted.2,3 EoE was mostly diagnosed in children in the past and was rare in adults. Recently, the incidence in adults has been increasing.4 The main symptoms in children are feeding difficulty, nausea and vomiting, abdominal pain, heartburn, and sometimes failure to thrive. In contrast, the main symptoms in adults are dysphagia, food impaction, and sometimes heartburn.1,5,6 Chest pain is one of the symptoms for EoE, but it can also be accompanied by coronary heart disease. This paper reports a case of EoE with angina pectoris, a major symptom of chest pain.
Go to : 
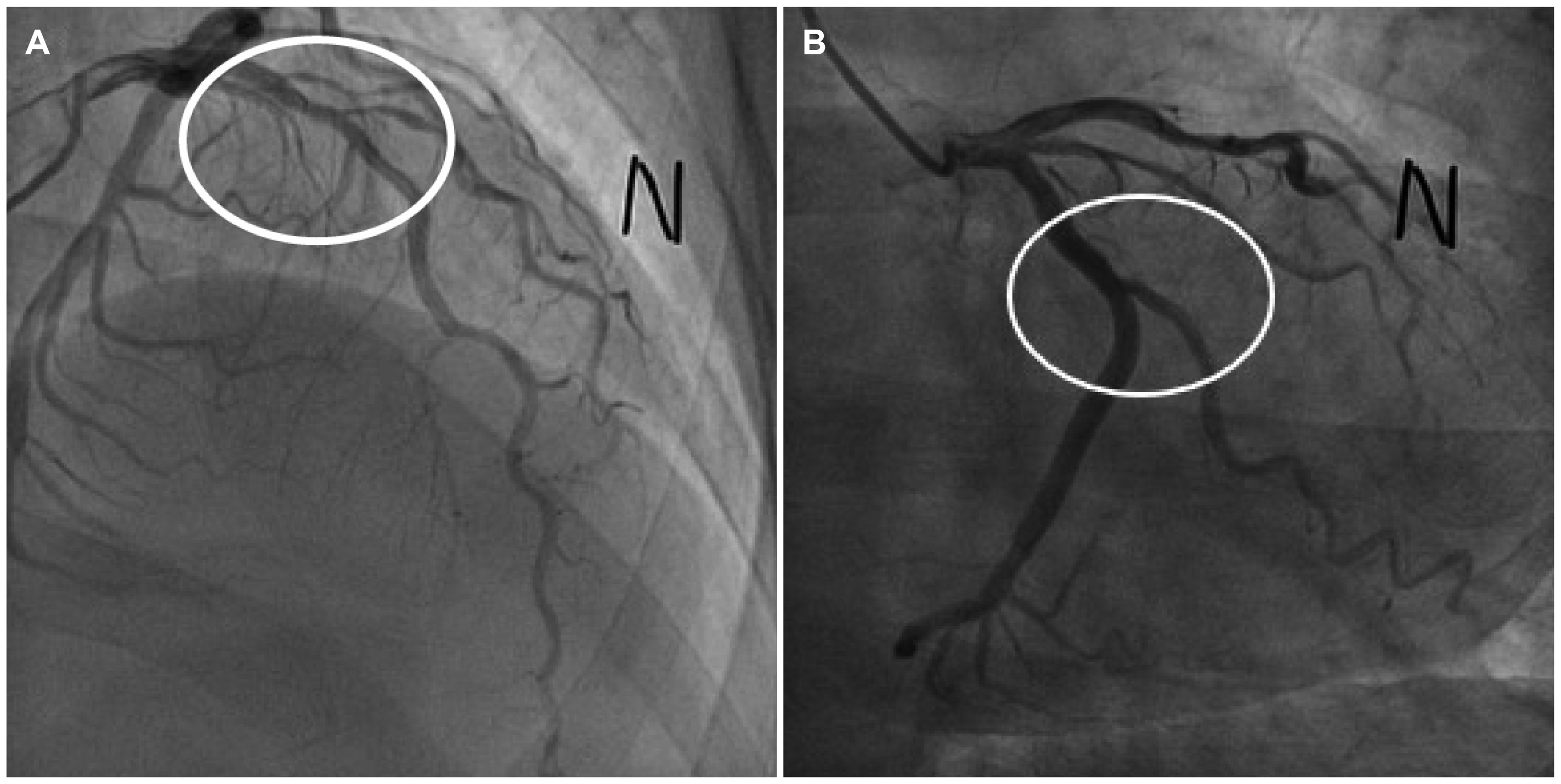
A 45-year-old male visited the emergency room with chest pain for one day. The chest pain was dull and accompanied by stuffiness that appeared to fill his neck. He had postprandial epigastric discomfort and a belching sensation for one month, but he was under observation without treatment. At the time of admission, his vital signs were as follows: blood pressure 170/90 mmHg, pulse 86 beats per minute, respiratory rate 20 times per minute, and temperature 36.5℃. He had no history of chest trauma. The physical examination and chest X-ray were unremarkable. No specific abnormal findings were found on the electrocardiogram. On laboratory testing, the white blood cell count of 9,560/μL (segmental neutrophil 68.8% and eosinophil 0.2%) and CRP count of 0.684 mg/dL (0-0.075) were high, but the other results were normal. In terms of the cardiac marker, the creatine kinase-MB (CK-MB) was normal at 2.1 U/L (0.6-6.3), but Troponin-I was elevated slightly to 48 mg/dL (0-40). His symptoms were regarded as the atypical chest pain of angina. Therefore, he was initially notified to the department of cardiology. A transthoracic echocardiogram was performed, and no abnormal findings were found. Coronary angiography was performed, which confirmed a 40-45% stenosis in the proximal to the middle part of the left anterior descending artery and mild discrete concentric stenosis (30%) in the obtuse marginal branch of the left circumflex artery (Fig. 1). The patient was diagnosed with mixed angina pectoris, and antianginal medication therapy was started.
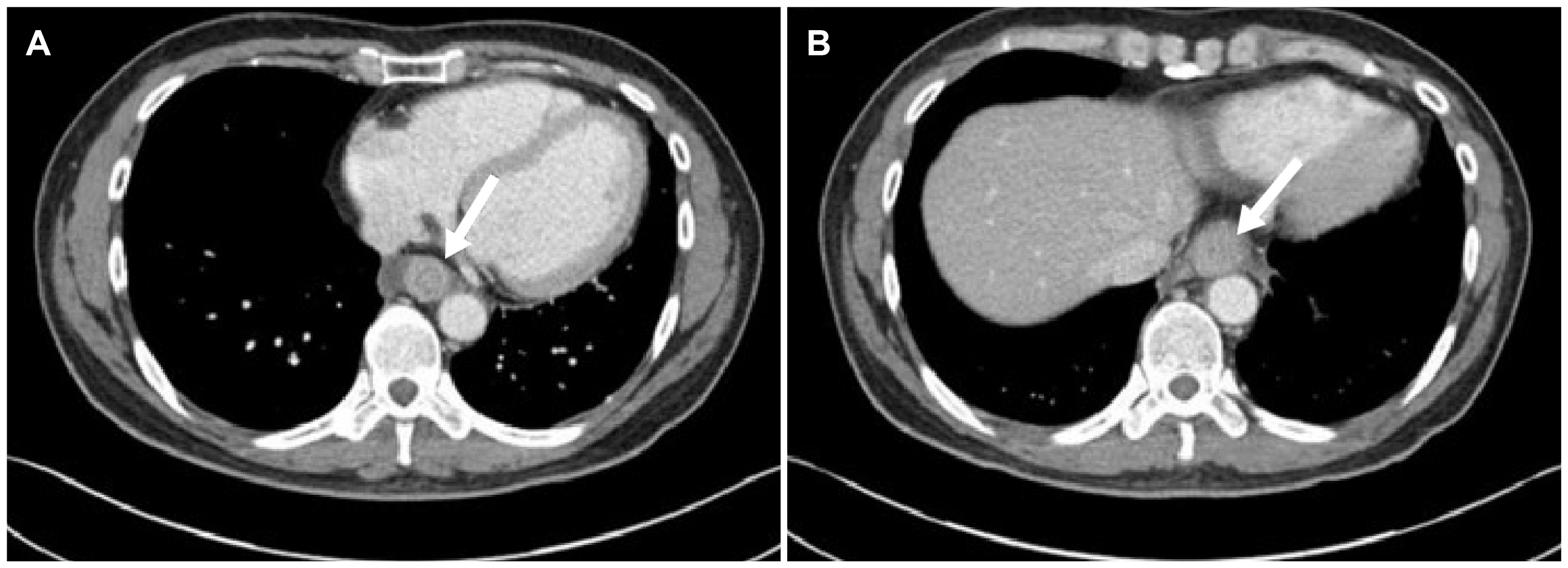
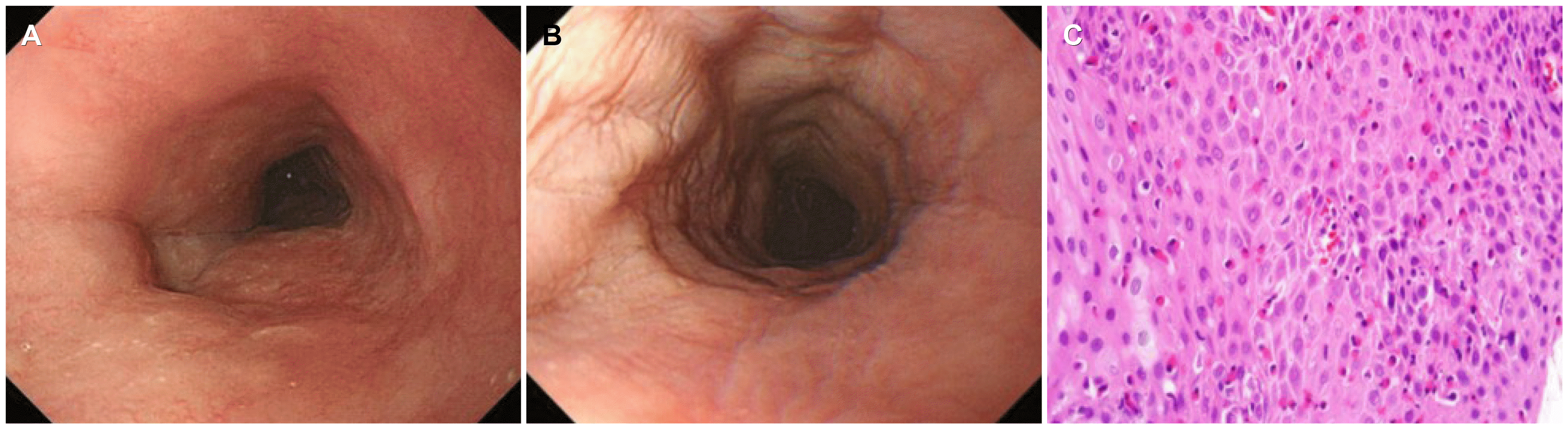
Despite the antianginal medication, the symptoms persisted without improvement. Thus, a chest CT scan was performed, which revealed diffuse wall thickening on the middle to lower esophagus (Fig. 2). An esophagogastroduodenoscopy (EGD) was then performed to eliminate esophageal diseases, such as esophageal malignancy. EGD revealed edematous mucosa and linear furrows on the middle to lower esophagus without an esophageal malignancy or reflux esophagitis (Fig. 3A, B). Esophageal biopsies were conducted on two specimens in the lower and middle esophagus under the suspicion of EoE. The histological examination revealed chronic active esophagitis with many eosinophil infiltrations of more than 90 eosinophils per high-power field (HPF) (Fig. 3C). The patient was diagnosed with EoE based on the examination results. Initially, the patient was treated with 30 mg lansoprazole, a proton pump inhibitor (PPI), twice daily. After two weeks of PPI treatment, he showed a 50% treatment response. Double dose lansoprazole was used continuously, and further examinations were performed to identify the other combined diseases.
In the diagnosis process, high-resolution esophageal manometry was performed to identify the esophageal motility disorders. The result of high-resolution esophageal manometry was normal lower sphincter relaxation, with 50% of failed peristalsis. Therefore, he was diagnosed with ineffective esophageal motility according to the Chicago Classification version 3.0.7
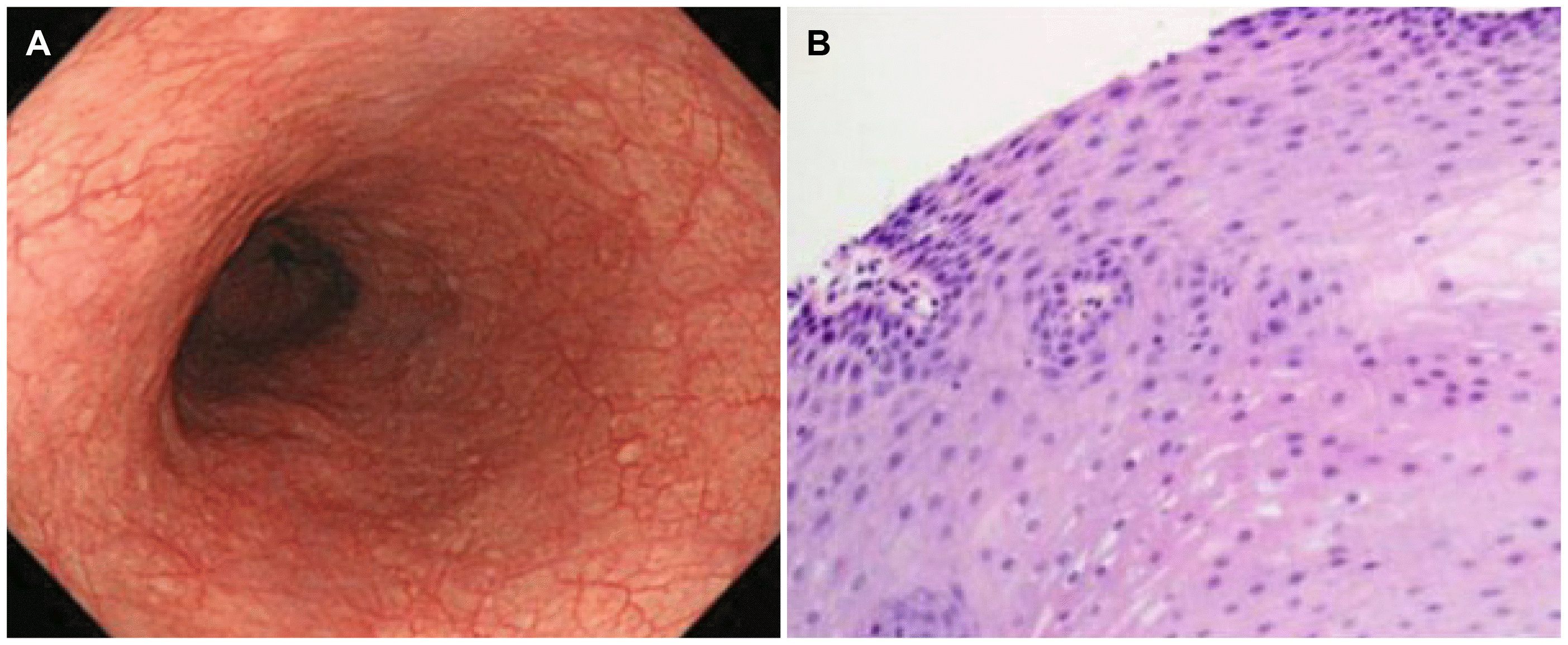
Allergic evaluations were also conducted to determine if the patient had allergic diseases. A 20% drop in forced expiratory volume 1 (FEV1) was high at 4.595 mg/mL in the methacholine bronchial challenge test. The total immunoglobulin E (IgE) was very high at 3.0, and the peach and Dermatophagoides pteronyssinus tick were high at 2.1 and 2.4 in the Multiple Thread Allergosorbent Test (MAST), respectively. The patient was diagnosed with asthma through the results, and the patient was given inhaled corticosteroid (fluticasone), leukotriene receptor antagonist (montelukast 10 mg), and antihistamine (loratadine 10 mg). After eight weeks, his chest pain symptoms had resolved completely. Low dose PPI (15 mg lansoprazole) was used for one month more after symptom improvement. Three months later, follow-up EGD revealed the disappearance of the linear furrows and mucosal edema (Fig. 4A). A histopathologic examination showed a marked decrease in eosinophil counts of less than five eosinophils per HPF (Fig. 4B). The patient was stable without recurrence at the one-year follow-up.
Go to : 
EoE is a chronic inflammatory disease diagnosed based on the symptoms of esophageal dysfunction and at least 15 eosinophils per HPF on esophageal biopsy after evaluation for other causes of esophageal eosinophilia.1,8 The endoscopic findings are also crucial for an accurate diagnosis of EoE. The typical endoscopic findings of EoE are the longitudinal furrows, white plaques, fixed/transient concentric rings, and edematous mucosa. The additional endoscopic findings are stricture, small/narrow-caliber esophagus, and crepe paper- like appearance. Longitudinal furrows and rings are the most common endoscopic findings,9 and EGD also identified furrows and edema in the present case. Edema and the fragility of the esophageal mucosa cause furrows and crepe paper esophagus. After all, chronic esophageal inflammation causes the esophageal mucosa to fibrosis and produces fixed rings, strictures, and narrow-caliber esophagus.
Current incidence estimates range from 5-10 cases per 100,000, and current prevalence estimates range from 0.5 to one case per 1,000 globally.10 This is growing rapidly in Western countries but rarely in Asian countries. In Korea, the first case of EoE was reported in 2002, and several cases have been described since 2008.11-13 Kim et al.2 reported that the diagnostic rate of EoE increased from 0.29 to 7.99 per 1,000 esophageal biopsies over the past 12 years (2006-2017) at one Korean institution. There are disagreements as to whether the actual prevalence is increasing or simply increased because of the new awareness that had previously been overlooked.
The mechanism of eosinophilic esophagitis is unclear, but it is associated with allergy. The present patient was also confirmed with asthma. Globally, the rates of allergic rhinitis, asthma, and eczema in EoE patients are 40% to 75%, 14% to 70%, and 4% to 60%, respectively.6 Kim et al.2 reported that allergic profiles of 72 patients diagnosed with EoE in a center in Korea 2006-2017 were 6.7% for allergic rhinitis, 12.5% for asthma, 4.2% for food allergy, and 1.4% for atopic dermatitis. Overall, approximately 53-73% of patients with EoE test positive on the skin prick test, related to IgE medicated exogenous allergic reaction.2 On the other hand, other patients with a negative skin prick test had an endogenous non-allergic response that was unrelated to IgE.2 The factors of the T helper 2 mediated immune response, interleukin-5, interleukin-13 and eotaxin are related to esophageal eosinophil infiltration, eventually damaging the esophagus tissue and causing an inflammatory response.6,14,15
The symptoms of EoE include feeding difficulty, nausea and vomiting, abdominal pain, heartburn, and failure to thrive in children. The symptoms of children usually appear differently depending on their age, and many symptoms appear together.1 In contrast, the main symptoms of EoE in adults are dysphagia and food impaction. In addition, EoE can also be combined with chest pain. Kinoshita et al.16 conducted a systemic review of the original study and case report published from 1980 to 2015 in 23 Asian countries, including Korea and Japan. Most symptoms confirmed by dysphasia (43.6%) and other symptoms included heartburn (22%), epigastric pain (11%), vomiting (6%), chest pain (5%), food impaction (4%), regurgitation (3%), and abdominal pain (2.8%).16 Therefore, chest pain was the fifth most common symptom in Asian adults. A retrospective review by Achem et al.17 reported that 24 out of 171 non-cardiac chest pain patients (14%) were diagnosed with EoE. Patients with EoE had significantly higher rates of male (71% vs. 34%), allergy (29% vs. 12%), endoscopic finding with rings (54% vs. 22%), and furrows (21% vs. 1%) compared to normal subjects.17 These results are in close agreement with the present patient. Kahn et al.18 reported 10 cases of EoE complaining of exercise-induced chest pain. Two of the 10 patients complained of chest pain without other symptoms. Chest pain tends to be approached as coronary heart disease in the clinical field, but it is not uncommon in EoE. In rare cases, as in the current patient, EoE and angina pectoris can occur concurrently.
The goals of EoE treatment are to relieve the symptoms and improve histologic inflammation. The treatments of EoE include elimination diet therapy, pharmacologic therapy, and endoscopic interventions for esophageal stricture.1 PPIs are the first-line pharmacological therapy. Recent guidelines for increasing efficacy recommend high dose PPI twice daily for eight weeks.19 In addition, after six to 12 weeks of initial therapy, follow-up endoscopy with a biopsy is recommended.19,20 Topical steroids (fluticasone, budesonide) may be offered as an effective first-line anti-inflammatory therapy. Furthermore, fluticasone propionate can be used as a metered-dose inhaler. The appropriate dose has not been established, and systemic steroids are not recommended.19 The current patient treated with high dose PPI therapy and asthma treatment with a fluticasone inhaler was combined for eight weeks. After eight weeks of treatment, the symptoms disappeared, and the inflammation remission was confirmed by follow-up endoscopic biopsy.
In this case, the improvement of the endoscopic and histologic findings of EoE was identified by treatment. This case is the EoE with angina by esophageal biopsies with coronary angiography, but the symptoms are presumed to be from EoE. This is because when the patient was treated with antianginal medication, he complained of persistent symptoms. The treatments of EoE improved the symptoms, including endoscopic and histologic features.
To the best of the authors’ knowledge, there has been no case similar reported. Although chest pain is the most common and important symptom of coronary heart disease, physicians need to consider other causes of chest pain, including EoE. Therefore, EoE may be considered when evaluating intractable unexplained chest pain, and consider esophageal biopsy during endoscopy, even if the endoscopic findings are normal.
Go to : 
REFERENCES
1. Furuta GT, Katzka DA. 2015; Eosinophilic esophagitis. N Engl J Med. 373:1640–1648. DOI: 10.1056/NEJMra1502863. PMID: 26488694. PMCID: PMC4905697.

2. Kim GH, Park YS, Jung KW, et al. 2019; An increasing trend of eosinophilic esophagitis in Korea and the clinical implication of the biomarkers to determine disease activity and treatment response in eosinophilic esophagitis. J Neurogastroenterol Motil. 25:525–533. DOI: 10.5056/jnm19066. PMID: 31587544. PMCID: PMC6786448.

3. Moawad FJ. 2018; Eosinophilic esophagitis: incidence and prevalence. Gastrointest Endosc Clin N Am. 28:15–25. DOI: 10.1016/j.giec.2017.07.001. PMID: 29129296.
4. Sgouros SN, Bergele C, Mantides A. 2006; Eosinophilic esophagitis in adults: a systematic review. Eur J Gastroenterol Hepatol. 18:211–217. DOI: 10.1097/00042737-200602000-00015. PMID: 16394804.

5. Furuta GT, Liacouras CA, Collins MH, et al. 2007; Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 133:1342–1363. DOI: 10.1053/j.gastro.2007.08.017. PMID: 17919504.

6. Liacouras CA, Furuta GT, Hirano I, et al. 2011; Eosinophilic esophagitis:updated consensus recommendations for children and adults. J Allergy Clin Immunol. 128:3–22. DOI: 10.1016/j.jaci.2011.02.040. PMID: 21477849.
7. Kahrilas PJ, Bredenoord AJ, Fox M, et al. 2015; The Chicago classification of esophageal motility disorders, v3. Neurogastroenterol Motil. 27:160–174. DOI: 10.1111/nmo.12477. PMID: 25469569. PMCID: PMC4308501.
8. Dellon ES, Liacouras CA, Molina-Infante J, et al. 2018; Updated international consensus diagnostic criteria for eosinophilic esophagitis:proceedings of the AGREE conference. Gastroenterology. 155:1022–1033. DOI: 10.1053/j.gastro.2018.07.009. PMID: 30009819. PMCID: PMC6174113.
9. Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. 2013; Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 62:489–495. DOI: 10.1136/gutjnl-2011-301817. PMID: 22619364.

10. Dellon ES, Hirano I. 2018; Epidemiology and natural history of eosinophilic esophagitis. Gastroenterology. 154:319–332.e3. DOI: 10.1053/j.gastro.2017.06.067. PMID: 28774845. PMCID: PMC5794619.

11. Kim JW, Park JS, Kim YH, et al. 2002; Secondary achalasia by eosinophilic esophagitis. Korean J Gastrointest Endosc. 25:198–202.
12. Lee BJ, Park HJ, Yoon HS, Kim HK, Kim HS. 2008; Three cases of eosinophilic esophagitis with dysphagia as a chief complaint. Clin Endosc. 36:145–149.
13. Park SB, Kim GH, Choi MK, et al. 2009; A case of eosinophilic esophagitis found incidentally during the evaluation of a gastric submucosal tumor. Clin Endosc. 39:212–216.
14. Arora AS, Yamazaki K. 2004; Eosinophilic esophagitis: asthma of the esophagus? Clin Gastroenterol Hepatol. 2:523–530. DOI: 10.1016/S1542-3565(04)00236-8. PMID: 15224275.

15. Hogan SP, Mishra A, Brandt EB, et al. 2001; A pathological function for eotaxin and eosinophils in eosinophilic gastrointestinal inflammation. Nat Immunol. 2:353–360. DOI: 10.1038/86365. PMID: 11276207.

16. Kinoshita Y, Ishimura N, Oshima N, Ishihara S. 2015; Systematic review:eosinophilic esophagitis in Asian countries. World J Gastroenterol. 21:8433–8440. DOI: 10.3748/wjg.v21.i27.8433. PMID: 26217096. PMCID: PMC4507114.
17. Achem SR, Almansa C, Krishna M, et al. 2011; Oesophageal eosinophilic infiltration in patients with noncardiac chest pain. Aliment Pharmacol Ther. 33:1194–1201. DOI: 10.1111/j.1365-2036.2011.04652.x. PMID: 21466568.

18. Kahn J, Bussmann C, Beglinger C, Straumann A, Hruz P. 2015; Exercise-induced chest pain: an atypical manifestation of eosinophilic esophagitis. Am J Med. 128:196–199. DOI: 10.1016/j.amjmed.2014.08.007. PMID: 25261012.

19. Lucendo AJ, Molina-Infante J, Arias Á, et al. 2017; Guidelines on eosinophilic esophagitis: evidence-based statements and recommendations for diagnosis and management in children and adults. United European Gastroenterol J. 5:335–358. DOI: 10.1177/2050640616689525. PMID: 28507746. PMCID: PMC5415218.

20. Cheng E, Zhang X, Huo X, et al. 2013; Omeprazole blocks eotaxin-3 expression by oesophageal squamous cells from patients with eosinophilic oesophagitis and GORD. Gut. 62:824–832. DOI: 10.1136/gutjnl-2012-302250. PMID: 22580413. PMCID: PMC3552049.

Go to : 




 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download