This article has been
cited by other articles in ScienceCentral.
Abstract
Gastric heterotopic pancreas is a relatively uncommon incidental finding. On the other hand, the presentation of gastric adenocarcinoma arising from a heterotopic pancreas is rare. This paper reports a case of gastric adenocarcinoma arising from a heterotopic pancreas that presented as a gastric outlet obstruction 10 years after the initial diagnosis of a suspicious submucosal tumor. Endoscopy revealed a pyloric stricture with prepyloric wall thickening and a complete gastric outlet obstruction. Abdominal and pelvic computed tomography exposed a severely distended gastric lumen at the antrum with heterogeneously enhancing circumferential wall thickening in the prepyloric antrum and pylorus. Because conservative treatment was ineffective and a malignancy could not be excluded, laparoscopic subtotal gastrectomy with a gastrojejunostomy was performed for histological confirmation and treatment. The histopathology diagnosis was advanced gastric carcinoma arising from heterotopic pancreatic tissue.
Go to :

Keywords: Pancreas, Stomach neoplasm, Adenocarcinoma, Gastric outlet obstruction
INTRODUCTION
Heterotopic pancreas is a relatively uncommon abnormality that can occur in any region in the gastrointestinal tract. The most common site is the stomach, particularly the antrum and prepyloric region. Although the typical endoscopic findings of a heterotopic pancreas have been described as a submucosal lesion with central umbilication, it is difficult to differentiate it from other diseases, such as gastrointestinal stromal tumors (GISTs), leiomyomas, or malignancies. In particular, gastric adenocarcinoma arising from a heterotopic pancreas is rare,
1-8 and the clinical course and management of gastric heterotopic pancreas are well established. There are no reports of gastric adenocarcinoma arising from a heterotopic pancreas that was confirmed after a long-term follow-up. This paper reports a case of gastric adenocarcinoma arising from a heterotopic pancreas presenting with a gastric outlet obstruction 10 years after the initial diagnosis of a suspicious submucosal tumor.
Go to :

CASE REPORT
A 75-year-old man was referred to the authors’ hospital in October 2019 with dyspepsia and nausea that had lasted for more than three weeks. The patient had lost 3 kg of body weight over 1 month but had an unremarkable medical history. The laboratory findings upon admission showed a white blood cell count of 7,000/mm3 (normal range, 4,000 to 10,000), hemoglobin 15.8 g/dL (14 to 18), amylase 37 U/L (28 to 100), lipase 31 U/L (13 to 60), CEA 5.7 ng/mL (0 to 4.3), and CA 19-9 37 U/mL (0 to 34). No specific abnormal findings were found except for increased CEA and CA 19-9 levels.
The patient was diagnosed with a suspicious submucosal tumor on the pyloric ring at another hospital in 2008 (
Fig. 1). The endoscopy findings revealed a lobulated lesion, approximately 2 cm in size, with central umbilication. The EUS findings revealed an indistinct, heterogeneous, and intermediate hypoechoic lesion involving the second and third layers. The lesion was considered to be a heterotopic pancreas on both the endoscopic and EUS findings. No abnormal findings were seen on the periodic follow-up endoscopy until three years ago (
Fig. 2).
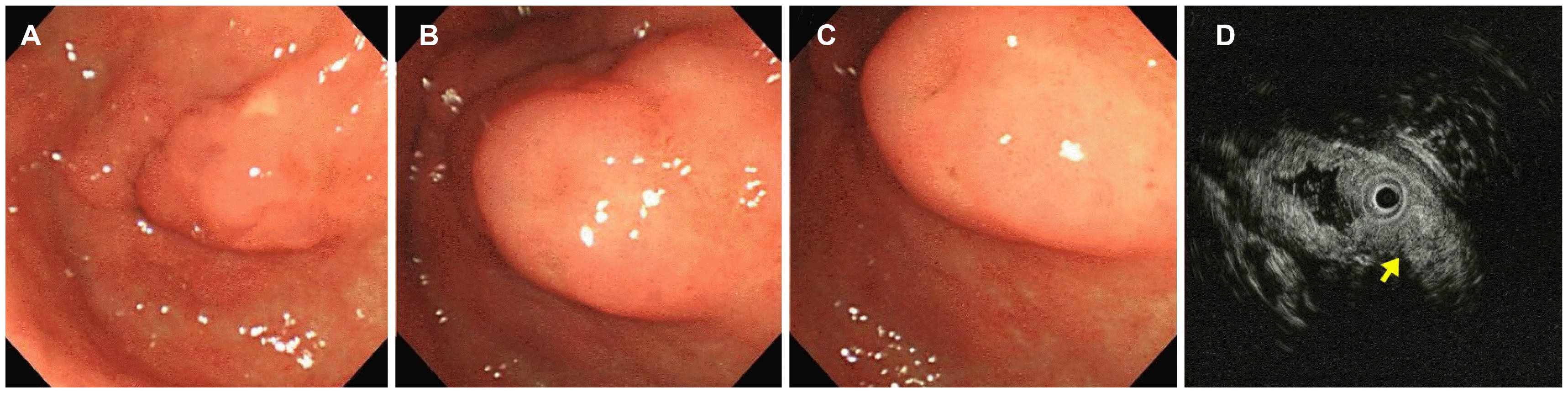 | Fig. 1Endoscopic and EUS images in 2008. (A-C) Endoscopic images showing a lobulated lesion with central umbilication. (D) EUS image showing an indistinct, heterogeneous, and intermediate hypoechoic lesion involving the second and third layers of the stomach (arrow). EUS, endoscopic ultrasonography. 
|
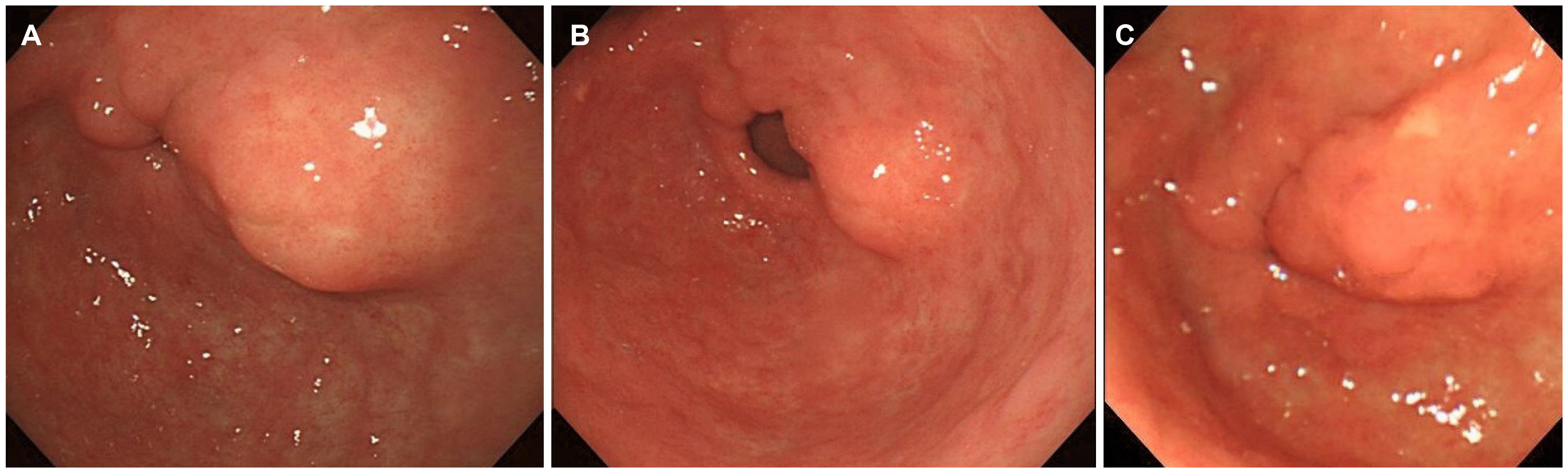 | Fig. 2Endoscopic images on periodic follow-up endoscopy (A) 2011, (B) 2014, and (C) 2016. 
|
Endoscopy performed at the authors’ hospital revealed an approximately 3 cm sized submucosal lesion and pylorus narrowing with prepyloric wall thickening. A complete gastric outlet obstruction was noted, and the endoscope could not pass through the stricture (
Fig. 3A). No lesions, such as ulcers or inflammation, were observed on the mucosal surface, and a biopsy was performed on the mucosa around the stricture. The biopsy results were reported as chronic active gastritis with intestinal metaplasia. A follow-up endoscopic biopsy was performed five days later, and the histologic diagnosis was chronic gastritis with no evidence of a malignancy. Abdominal and pelvic CT revealed a severely distended gastric lumen at the antrum with heterogeneously enhancing circumferential wall thickening in the pre-pyloric antrum and pylorus, suggesting stomach cancer (T3) (
Fig. 3B). A differential diagnosis of acute heterotopic pancreatitis and adenocarcinoma arising in a heterotopic pancreas was considered due to the increased tumor size and the resulting gastric outlet obstruction during the follow-up period. On the other hand, the patient had no symptoms of acute severe abdominal pain or elevated pancreatic enzyme levels. Conservative treatment with an intravenous proton pump inhibitor and fasting for eight days was ineffective, and abdominal CT did not exclude a malignancy. Therefore, a surgical approach was considered. A laparoscopic subtotal gastrectomy with gastrojejunostomy was performed for histological confirmation and treatment.
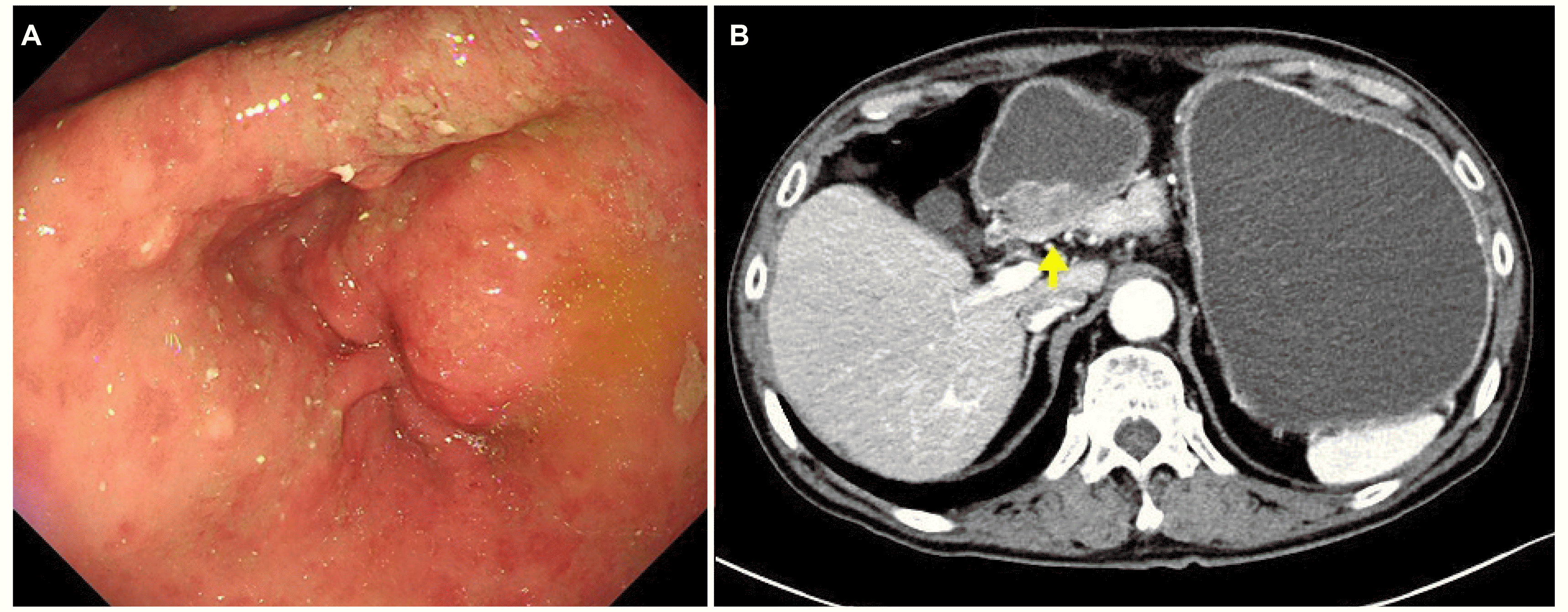 | Fig. 3Endoscopic and contrast-enhanced abdominal computed tomography (CT) images at 2019. (A) Endoscopic image showing an approximately 3 cm sized submucosal lesion and pylorus narrowing. (B) CT image showing a severe distended gastric lumen at the antrum with heterogeneously enhancing circumferential wall thickening in the pre-pyloric antrum and pylorus (arrow). 
|
The gross findings of the subtotal gastrectomy specimen revealed a mass with erosion in the distal antrum. The cut sections revealed an ill-defined whitish and solid mass with small cystic spaces (
Fig. 4A). The mass measured 3.5×1.7×1.3 cm and involved the entire thickness of the gastric wall. Microscopically, the tumor contained malignant glands that developed in the heterotopic pancreas (
Fig. 4B). The heterotopic pancreas was located in the submucosa and muscularis propria. The mucosa over the tumor was intact, except in the regional area of the erosion. The non-neoplastic heterotopic pancreatic tissue showed ducts and acini (
Fig. 4C). Dilatation of the ducts was also observed. The tumor was characterized by a random distribution of well-to-poorly differentiated glandular structures. The tumor cells were medium to large with eosinophilic cytoplasm and marked nuclear pleomorphism. The majority of the dilated ducts showed papillary features of the neoplastic epithelium as well as transitional areas between the non-neoplastic epithelium and dysplasia. The high grade pancreatic intraepithelial neoplasia (PanIN) was characterized by papillary elements lined with cells with significant cytologic atypia, and low-grade PanIN showed a duct lined with a flat epithelium composed of tall columnar mucin-producing cells with no cytologic atypia (
Fig. 4D). Lymphovascular invasion was identified. The tumor penetrated the subserosal connective tissues without invading the visceral peritoneum. The pathological diagnosis of an adenocarcinoma (T3 N0 stage) arising from the heterotopic pancreas was concluded.
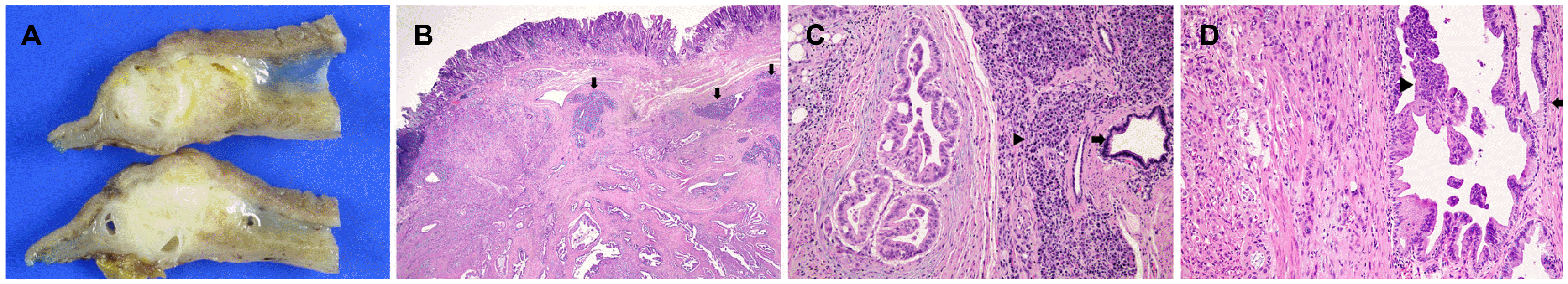 | Fig. 4Gross and histopathology findings. (A) Cut sections of the whitish solid mass that invaded the subserosa. (B) The tumor showed well to poorly differentiated glands in the heterotopic pancreas. Non-neoplastic heterotopic pancreas (arrows) consisting of ducts and acini in the submucosa on the upper right side (H&E stain, ×1.25). (C) Photomicrograph of histopathology specimen shows heterotopic pancreatic tissue consisting of ducts (arrow) and acini (arrowhead). Well-differentiated adenocarcinoma is noted (on the left) (H&E stain, ×100). (D) High grade pancreatic intraepithelial neoplasia (PanIN) is characterized by papillary elements lined by cells with significant cytologic atypia (arrowhead) and low-grade PanIN (arrow) shows duct lined by flat epithelium composed of tall columnar mucin-producing cells with no cytologic atypia. Moderately differentiated malignant glands infiltrating into the fibrotic stroma (on the left side) (H&E, ×200). 
|
The patient received chemotherapy with capecitabine and intravenous oxaliplatin after surgery. Follow-up CT and endoscopy were scheduled 6 months after surgery.
Go to :

DISCUSSION
Heterotopic pancreas is often asymptomatic, and subsequent cystic formation, pancreatitis, and malignant changes can occur.
9,10 On the other hand, gastric adenocarcinoma arising from the heterotopic pancreas is rare, and there have been no previously reported cases of gastric adenocarcinoma arising from the heterotopic pancreas, which were confirmed after a long-term follow-up.
The typical endoscopic findings of a heterotopic pancreas are a well-circumscribed submucosal lesion with central umbilication. On the other hand, as umbilication is noted in less than half of cases, it is difficult to differentiate heterotopic pancreas from other diseases, such as GIST, leiomyoma, or submucosal gastric carcinoma. The EUS findings of a heterotopic pancreas are a hypoechoic or intermediate echogenic heterogeneous lesion with indistinct margins. This most commonly arises from the third or fourth layer, or a combination of the two layers.
For a diagnosis of carcinoma arising from a heterotopic pancreas, the following three criteria have been proposed.
11 First, the carcinoma must be within or close to the ectopic pancreas. Second, a transitional area between the pancreatic structures and carcinoma must be present. Third, non-neoplastic pancreatic tissue must be comprised of well-developed acini or ductal structures. The present case meets the above three criteria (
Fig. 4).
The malignant transformation of a gastric heterotopic pancreas is rare but has been reported in the literature. In addition, clinicians need to consider the possibility of a malignant transformation. The early diagnosis of malignant changes arising in the gastric heterotopic pancreas may be difficult because the surface of the lesion is usually covered with normal mucosa. Although various diagnostic techniques yield results, EUS-guided fine needle aspiration (FNA) or fine needle biopsy (FNB) are useful methods for making a histologic diagnosis of submucosal lesions.
12,13 EUS-FNA has been reported to be helpful in the diagnosis of heterotopic pancreas.
14 A periodic follow-up by endoscopy or EUS should be considered because malignant changes from the heterotopic pancreas can occur after more than 10 years, as in this case. In addition, EUS-FNA or FNB may be useful for an early diagnosis of the malignant transformation of heterotopic pancreas if the size of the lesion increases or shows atypical changes.
The guidelines for the management of gastric heterotopic pancreas have not been well established. Gastric heterotopic pancreas is usually asymptomatic and requires no treatment. Histologic confirmation through a resection is required in cases with a high risk, an increasing size, and symptoms, such as ulceration, bleeding, and pyloric obstruction. Unlike GIST, which sets a size of approximately 2 cm or more as high-risk criteria,
15 a heterotopic pancreas does not have a clear standard for size in the high-risk criteria. On the other hand, as the size of the heterotopic pancreas was more than 2 cm in most cases reported previously as a gastric adenocarcinoma arising from a heterotopic pancreas,
8 the size of the lesion may need to be considered as an important risk factor, such as in GIST.
Surgery is frequently needed to make a definitive diagnosis and plan further treatment because of the difficulty in differentiating a heterotopic pancreas from other diseases, such as GIST, leiomyoma, neuroendocrine tumors, or other malignancies. Although a surgical resection is usually recommended for the treatment of symptomatic heterotopic pancreas, an endoscopic resection, such as endoscopic submucosal dissection or mucosal resection, can be considered after taking into account the size and location of the lesion in cases with benign features. Periodic follow-up endoscopy or EUS is recommended if there are no symptoms.
In conclusion, although the incidence of gastric adenocarcinoma arising from a heterotopic pancreas is rare, a careful diagnostic and therapeutic approach should be considered in patients with gastric heterotopic pancreas. In addition, clinicians need to consider the possibility of a malignant transformation.
Go to :








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download