INTRODUCTION
Colorectal cancer (CRC) was the third leading cause of worldwide cancer-related death in 2018, with an age-standardized mortality rate of 8.9 per 100,000.
1 Approximately one-quarter of patients with newly diagnosed CRC have metastatic lesions and half of the patients eventually proceed to metastatic CRC.
2 Despite the development of new CRC treatments, the overall survival (OS) of patients with metastatic CRC is still less than 30 months.
3
CRC is a heterogeneous and complex disease resulting from the accumulation of genetic and epigenetic alterations.
4,5 The molecular heterogeneity of CRC varies according to the tumor location, and recent biological and clinical data indicate that the molecular pathway differs significantly between right-sided and left-sided CRC.
6 In terms of an embryological origin, right-sided and left-sided CRC derives from the embryonic midgut and embryonic hindgut, respectively.
7 Right-sided CRC is characterized more frequently by high microsatellite instability, CpG island methylation, B-type Raf Kinase (BRAF) mutations, and poor differentiation compared to left-sided CRC.
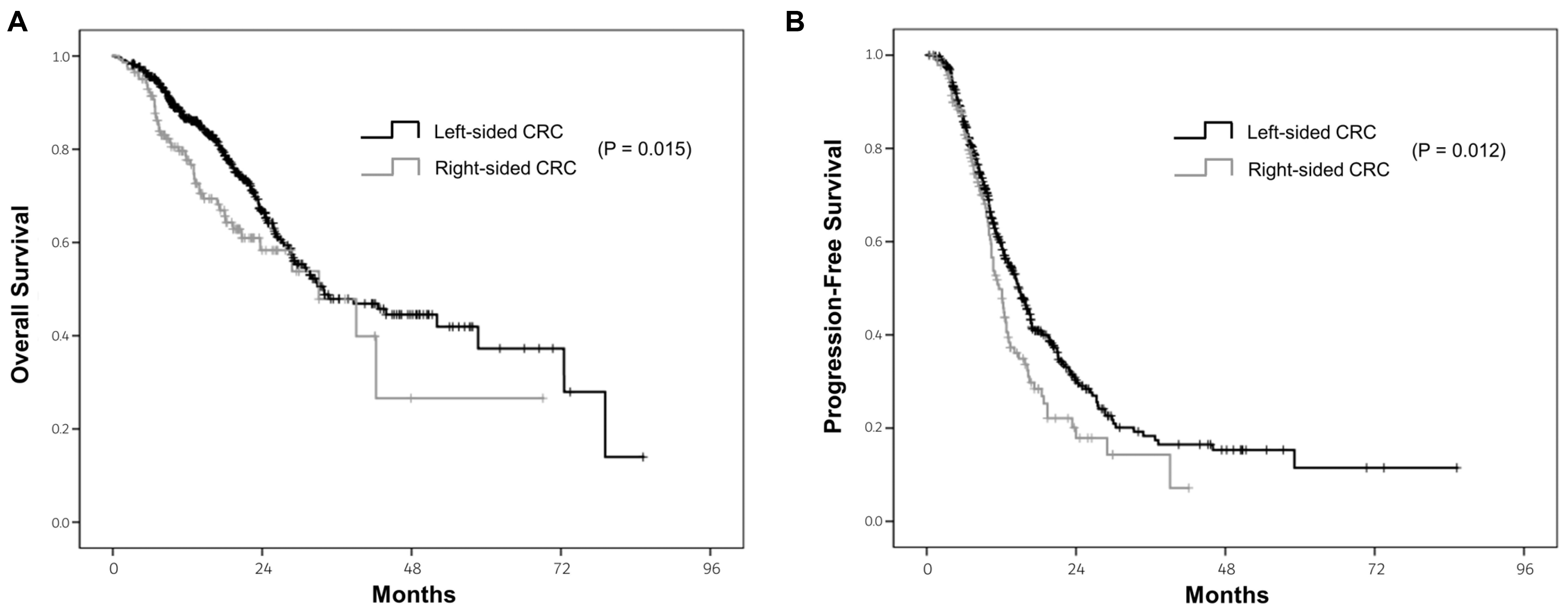
8,9 These differences result in different clinical behaviors, with right-sided CRC showing a poorer prognosis.
10,11
Patients with a resectable primary tumor and metastatic lesions can be treated with surgery, and the removal of both the primary tumor and metastatic lesions is associated with favorable outcomes. On the other hand, the survival outcomes of a palliative primary tumor resection in patients with unresectable metastatic CRC remain unclear. A population-based analysis of 37,793 metastatic CRC patients showed that patients who underwent a palliative primary tumor resection had a better overall and disease-free survival.
12 On the other hand, an observational cohort study, including 15,154 metastatic CRC patients, showed that a palliative primary tumor resection was not associated with improved survival compared to systemic chemotherapy.
13 The current National Comprehensive Cancer Network (NCCN) guideline recommendations are as follows: consider a colon resection only if there is an imminent risk of obstruction, significant bleeding, perforation, or other significant tumor-related symptoms.
14
In this study, it was hypothesized that a palliative primary tumor resection is an important factor affecting the prognosis of patients with metastatic CRC, and it differs according to the primary tumor location. This study investigated the long-term outcomes based on the primary tumor location in patients with metastatic CRC and compared the survival outcomes based on a palliative primary tumor resection.
Go to :

SUBJECTS AND METHODS
1. Patients and data collection
The medical records of patients diagnosed with metastatic CRC at Kosin University Gospel Hospital (Busan, Korea) between January 2000 and June 2018 were reviewed retrospectively; patients with histologically confirmed colonic or rectal adenocarcinoma were included. Patients whose medical records did not include the clinicopathological and follow- up data, and patients undergoing surgery for both the primary tumor and metastatic lesions were excluded. This study was approved by the Institutional Review Board of Kosin University Gospel Hospital (KUGH 2020-03-036).
The following detailed clinical data were collected: patient age, sex, BMI, blood type, history of smoking or alcohol, family cancer history, performance status, co-morbidities, primary tumor location and metastatic sites, histopathology, biomarkers including microsatellite instability (MSI), KRAS and NRAS, tumor stage, laboratory findings, and treatment outcomes, including chemotherapy, radiotherapy, and surgery.
2. Classification of primary tumor location
The clinicopathological factors were evaluated according to the primary tumor location. Primary tumors originating in the appendix, cecum, ascending colon, hepatic flexure, and transverse colon were classified as right-sided CRC. Primary tumors originating in the splenic flexure, descending colon, sigmoid colon, and rectum were classified as left-sided CRC. Patients with tumors originating on both sides were classified as indeterminate- sided and were excluded.
3. Definition of tumor resection
A palliative primary tumor resection was defined as the removal of the primary tumor without removing the metastatic lesions. The palliative primary tumor resection included a left anterior resection, anterior resection, Miles operation, Hartmann’s operation, left hemicolectomy, segmentectomy, right hemicolectomy, and ileocecectomy with a lymph node dissection. No tumor resection was defined when the primary tumor was not removed.
4. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics version 24.0 (IBM Co., Armonk, NY, USA). A student’s t-test and chi-square test were performed for the continuous and categorical variables, where appropriate. The OS was measured from the date of the CRC diagnosis to the date of death or final follow-up. The progression-free survival (PFS) was measured from the date of the CRC diagnosis to the date of recurrence or final follow-up. CRC recurrence was diagnosed based on the radiological and endoscopic histopathological data. Kaplan-Meier curves were used to construct survival curves based on cumulative incidences and compared using a log-rank test. The Cox proportional hazards regression model was used to assess the factors affecting the OS and PFS. p values <0.05 were considered significant.
Go to :

DISCUSSION
These findings show the prognostic roles of primary tumor location and primary tumor resection in patients with metastatic CRC. The data suggest that a primary tumor resection can improve the OS in patients with metastatic CRC, regardless of the primary tumor location.
In the present study, patients with right-sided CRC had several characteristics that differed from those with left-sided CRC, which included older age, more female patients, BMI 23 kg/m
2 or higher, poorly differentiated/signet ring cell/mucinous histology, and peritoneal metastasis. These findings are consistent with other recent data,
6,8,9 which likely result from different molecular carcinogenesis pathways between right- and left-sided CRC. The primary tumor location is associated with the prognosis in CRC patients, and generally, right-sided CRC has a poorer outcome.
15-17 The data shows that patients with right-sided CRC have poorer survival outcomes than those with left-sided CRC. In propensity score analysis, right-sided CRC was diagnosed at a more advanced state within stage IV disease and showed significantly poorer prognosis than left-sided CRC.
18 In this study, the characteristics of patients with right-sided CRC, including older age, more female patients, and more poorly differentiated/signet ring cell/mucinous histology, are considered as contributors to poorer survival benefits. A primary tumor location influences the selection of the chemotherapy regimen in patients diagnosed with metastatic CRC. A retrospective analysis of the CRYSTAL and FIRE-3 trials analyzed RAS wild-type populations. They showed that first-line FOLFIRI (irinotecan, fluorouracil, and leucovorin) plus cetuximab benefitted patients with left-sided CRC more than those with right-sided CRC.
7 A meta-analysis of first-line clinical trials, including PRIME, CRYSTAL, FIRE-3, and the CALGB/SWOG 80405 study, indicated that patients with RAS wild-type left-sided CRC had a significant survival benefit from the addition of an anti-epidermal growth factor receptor (EGFR) antibody to conventional chemotherapy.
19 Based on these results, the current NCCN guidelines recommend the addition of anti-EGFR antibody to conventional chemotherapy for metastatic CRC patients with KRAS/NRAS/BRAF wild type genes and left-sided tumors.
14
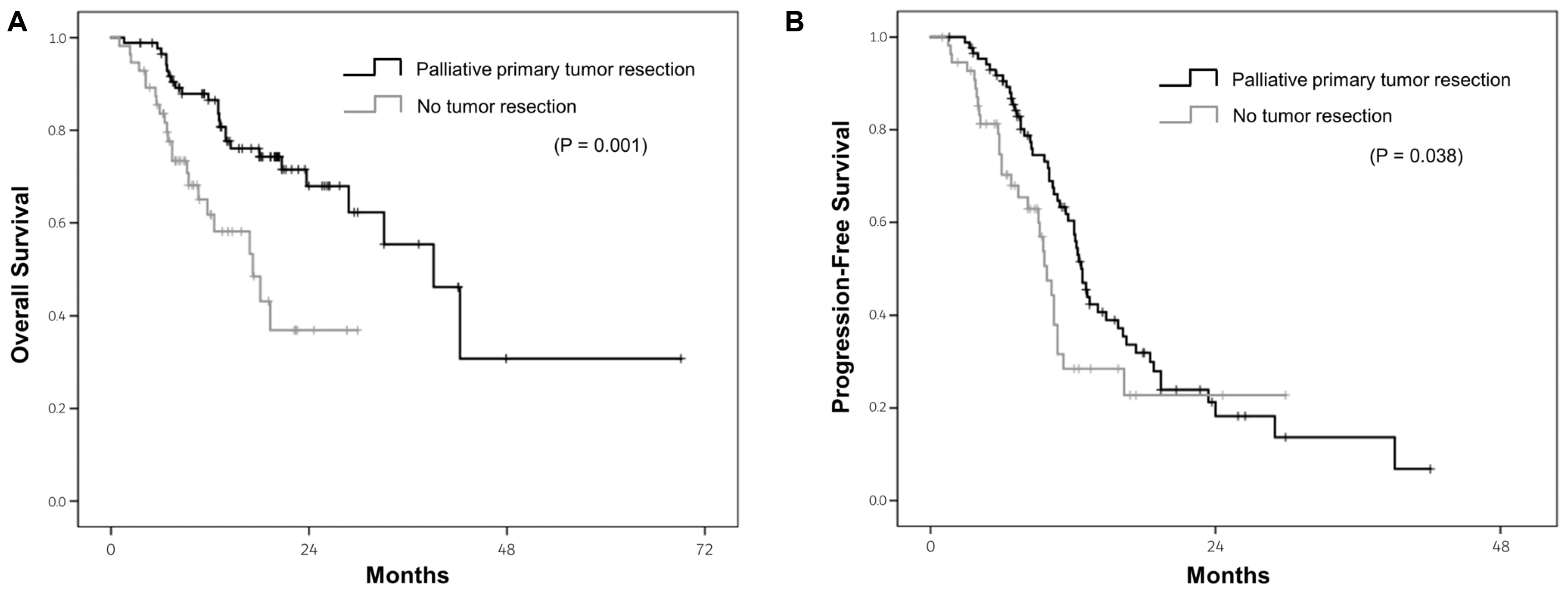
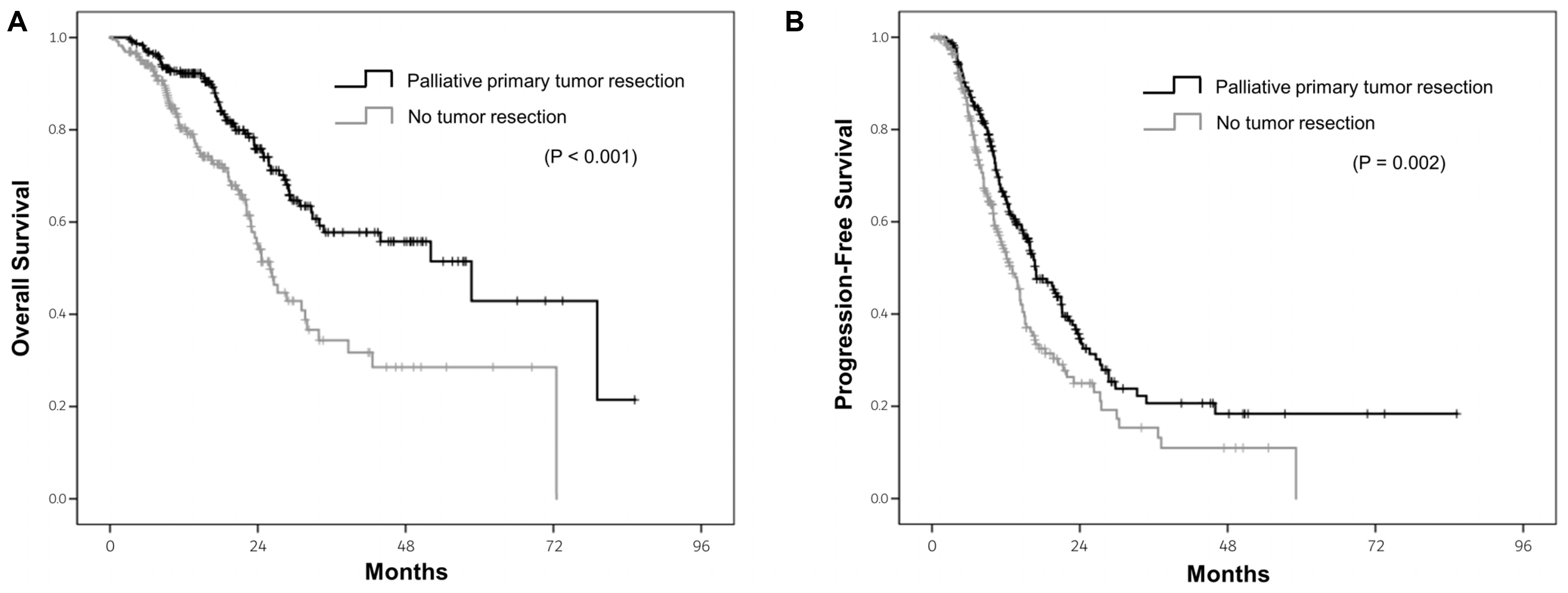
The data revealed a survival benefit of palliative primary tumor resection. In this study, a palliative primary tumor resection was performed more in patients with right-sided CRC than those with left-sided CRC (60.8% vs. 49.9%,
Table 3). Multivariate analysis revealed a palliative primary tumor resection to be an independent prognostic factor for a better OS, regardless of the primary tumor location. Surgical resection of the primary tumor is a curative treatment for CRC patients without metastasis. On the other hand, the role of primary tumor resection for CRC patients with metastases is unclear. A reduction of the tumor burden may lead to a better response to systemic therapy in patients with metastatic CRC.
20 A reduction of the tumor burden has a survival benefit for primary renal or ovarian tumors with metastatic lesions.
21,22 A recent study suggested that a primary tumor resection can prevent micro-metastases to the liver parenchyma by increasing the angiogenic markers, including vascular endothelial growth factor (VEGF) A, VEGF receptor 1, VEGF receptor 2, and placental growth factor.
23 On the other hand, some studies reported worsening liver or lung metastases after a primary tumor resection, which was attributed to the depletion of anti-angiogenic proteins, such as angiostatin and endostatin produced by the primary tumor.
24,25 Further studies will be needed to clarify the role of the primary tumor resection for patients with metastatic CRC.
This study had several limitations. First, this study had a retrospective, single-center design, which means the selection bias could not be avoided, even though an attempt was made to minimize bias by repeatedly reviewing the medical records. Second, the efficacy of an anti-EGFR antibody plus conventional chemotherapy depending on the location of the primary tumor could not be confirmed, because the number of tests for KRAS/NRAS and the proportion of patients treated with biologics were not high enough to assess the efficacy. Third, tumors originating from the transverse colon were classified as right-sided CRC for the convenience of analysis, but classifying transverse colon cancer as right-sided vs. left-sided is controversial. To overcome this limitation, further well-designed studies for the characterization of transverse colon cancer will be needed.
In conclusion, this study showed that the primary tumor location has a prognostic effect on patients with metastatic CRC. Moreover, a palliative primary tumor resection is a significant prognostic factor affecting better OS, regardless of the primary tumor location. Based on these results, a palliative primary tumor resection is a suitable option for patients with metastatic CRC to improve the long-term outcomes, regardless of the primary tumor location.
Go to :





 PDF
PDF Citation
Citation Print
Print



 XML Download
XML Download