INTRODUCTION
Ectopic pancreas, which is also known as heterotopic pancreas and accessory pancreas, refers to ectopic pancreatic tissue found frequently in the distal stomach, duodenum, or proximal jejunum and within the Meckel’s diverticulum, gallbladder, bile ducts, and minor and major papillae.
1 Although most patients with ectopic pancreas are asymptomatic, rare complications have been reported, such as upper gastrointestinal bleeding, gastric outlet obstruction, obstructive jaundice, intestinal obstruction, intussusception, and malignant transformation.
2,3 The typical endoscopic findings include a firm round or oval subepithelial lesion (SEL) with central dimpling or umbilication.
4,5 EUS provides useful information regarding the location, size, and echogenicity of a tumor. On the other hand, it is difficult to differentiate between ectopic pancreas and other SELs, particularly gastrointestinal stromal tumors, which have malignant potential.
6 Therefore, a histological diagnosis is required in some cases. As a diagnosis is usually difficult with specimens obtained using standard biopsy forceps, endoscopic techniques, including endoscopic mucosal resection (EMR), endoscopic submucosal dissection (ESD), and EUS-guided tissue sampling, have been developed to obtain tissue samples.
7-9 Sometimes, it is difficult to achieve a complete resection by EMR or ESD due to probable noninflammatory adhesion, which can occur during embryonic development, as explained by the aberrant primordium theory.
10 This study examined the EUS characteristics of gastric ectopic pancreas (GEP) confirmed by histological analysis of the specimens obtained by an endoscopic resection.
Go to :

SUBJECTS AND METHODS
1. Patients and study protocol
A total of 6,143 patients who underwent EUS for an evaluation of gastric SELs between January 2004 and July 2018 at Chonnam National University Hospital were identified retrospectively. Of these, 182 patients underwent an endoscopic resection. Finally, 31 patients with histologically confirmed GEP were identified. These patients underwent EUS before the endoscopic resection (
Fig. 1).
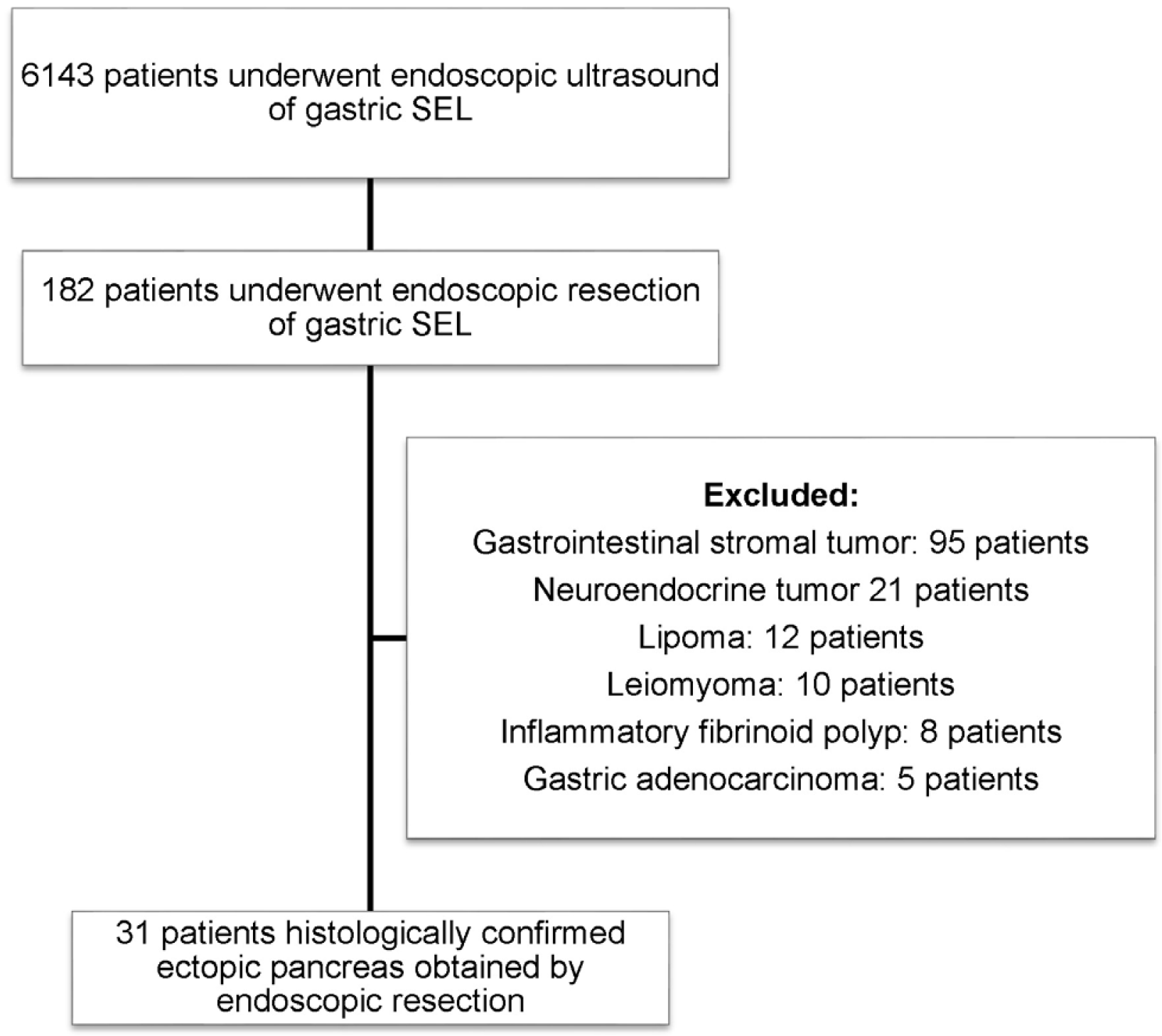 | Fig. 1Flow chart of the study. SEL, subepithelial lesion. 
|
2. Endoscopic and EUS findings
A EUS probe (UM-2R; Olympus, Tokyo, Japan) and probe driving unit (MAJ-935; Olympus) were initially used to map the lesions. The imaging frequency of the probe was 12 MHz. EUS was performed by an experienced endosonographer (J.S.R.). All examinations were performed under intravenous sedation using midazolam and propofol. The lesion was scanned after filling the stomach with deaerated water.
DH Kim and HS Lee recorded and reviewed the following EUS features for all lesions: 1) location, 2) gross shape (Yamada classification
11), 3) presence of central dimpling or umbilication on the surface, 4) maximal diameter, 5) layer of origin, 6) echogenicity (hypoechoic, hyperechoic or mixed), 7) homogeneity (homogeneous or heterogeneous), 8) distinctness of the border (distinct or indistinct), 9) presence of anechoic duct-like structures, 10) presence of hyperechoic foci with acoustic shadowing (suggestive calcification), and 11) muscularis propria (PM; fourth layer) thickening (PM layer thickening was compared with normal PM layer thickening [PM
ep/PM
normal]). Ectopic pancreas was classified as the superficial type (S-type) and deep type (D-type) based on the classification proposed by Park et al.
4 In the S-type, the lesion originated in the second or third layer. In the D-type, the lesion was in the third and fourth layers with or without extension into the fifth layer. Moreover, PM thickening was defined as “PM
ep/PM
normal ≥2” (
Fig. 2).
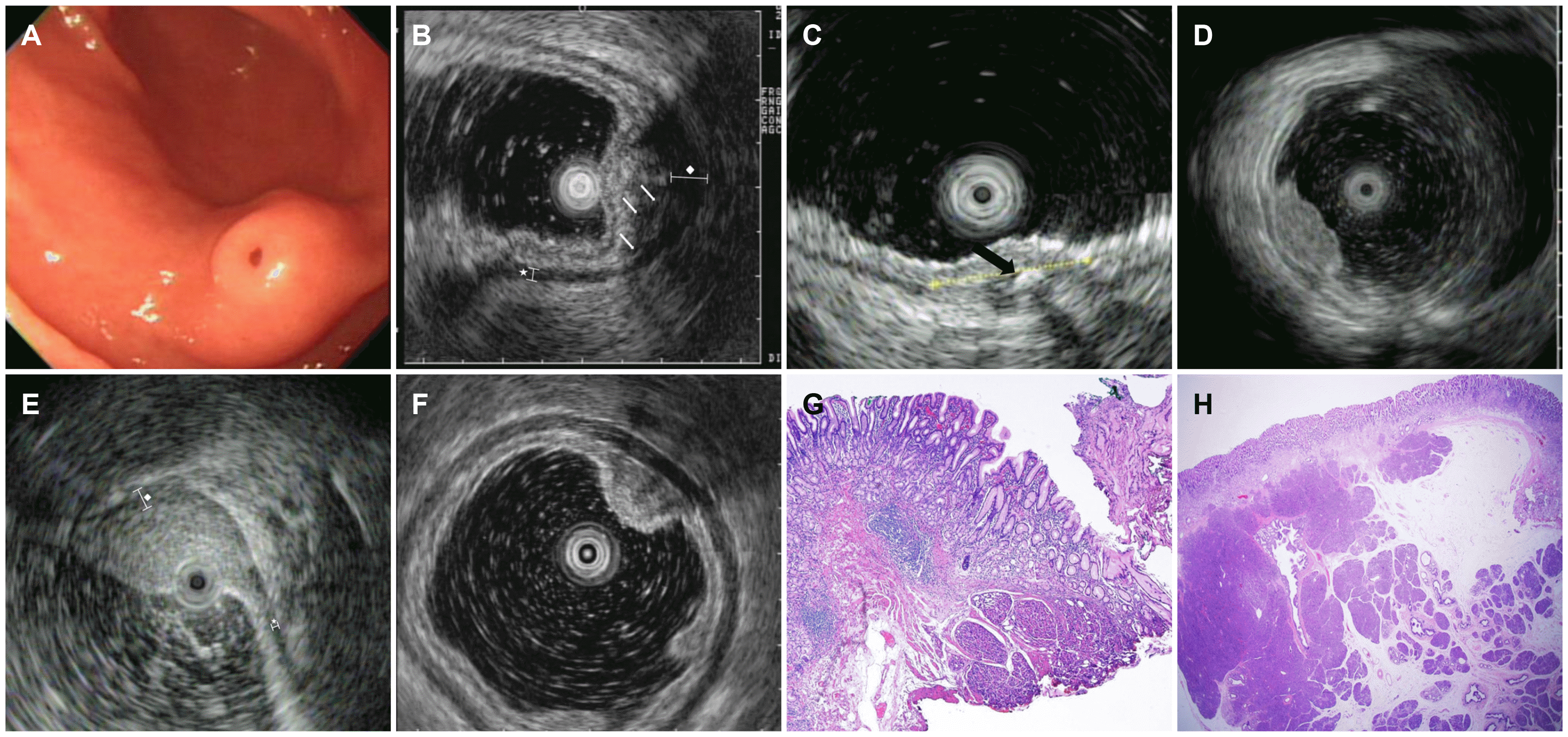 | Fig. 2Endoscopic and EUS features of an ectopic pancreas. (A) Endoscopic appearance of an ectopic pancreas indicating Yamada type II polypoid lesion with central dimpling. (B) EUS image of the same lesion shown in Fig. 2A indicting that the SM (3rd) and PM (4th) layers are involved. EUS image showing a lesion of mixed echogenicity, heterogeneity, and indistinct border. The white arrows indicate anechoic duct-like structures. The star (*) represents the diameter of normal PM (PMnormal), and the rhombus represents the diameter of the lesion with PM thickening (PMep). In this case, PM thickening “PMep/PMnormal ≥2” was observed. (C) EUS image showing the presence of hyperechoic foci with acoustic shadowing suggesting central calcification. The black arrow indicates hyperechoic foci. (D) EUS image showing ectopic pancreas involving the MM (2nd) layer. Lesions with hyperechoic echogenicity, heterogeneity, and distinct border without PM thickening can be observed. (E) EUS image showing the ectopic pancreas involving the SM (3rd) layer. A lesion with hyperechoic echogenicity, heterogeneity, and an indistinct border with PM thickening “PMep/PMnormal ≥2; the rhombus represents the diameter of the lesion with PM thickening ((PMep). The star (*) represents diameter of normal PM (PMnormal). (F) EUS image showing ectopic pancreas involving the MM (2nd) layer. Lesions with hypoechoic echogenicity, heterogeneity, and distinct border without PM thickening are observed. (G) Histopathology findings of S-type ectopic pancreas. The biopsy specimen of the stomach showed a heterotopic pancreas composed of acinar cells (H&E, ×20). Heterotopic pancreatic tissue located in the submucosal layer. (H) Histopathology findings of D-type ectopic pancreas. Histopathology image showing pancreatic tissue composed of acinar cells and ductal elements located in the submucosal and subserosal layers of the stomach (H&E, ×20). EUS, endoscopic ultrasonography; SM, submucosa; PM, muscularis propria; MM, muscularis mucosa. 
|
3. Endoscopic resection
Endoscopic resection was performed using two methods: EMR and ESD. EMR was performed using the injection-and-snaring technique. First, a saline injection with a small amount of epinephrine (0.025 mg/mL) and indigo carmine was used to lift the lesion. The lesion was then elevated by retraction using grasping forceps (FD-410LR; Olympus) that was passed through a polypectomy snare loop (SD-5L-1; Olympus). After snaring, the lesion was resected. ESD was performed by injection and dissection of the tumor. In ESD, the margins of the lesion were marked using the tip knife, and a submucosal injection of saline with a small amount of epinephrine and indigo carmine lifted the lesion. An initial circumferential incision was then made around the lesion, and the lesion was dissected using an electrosurgical knife (Dual Knife [KD-650L]; Olympus).
4. Definition
1) The procedure time was defined as the time from the first marking or the first injection to the achievement of hemostasis. 2) An en bloc resection was defined as a resection wherein the tumor was resected as a single piece. 3) Complete resection was defined as a resection wherein the tumor was removed in one piece (en bloc resection), and the horizontal/vertical margin was histologically free from the tumorous glands. And 4) adverse events were defined as complications, such as bleeding, perforation, or aspiration pneumonia, associated with the endoscopic resection.
5. Statistical analysis
Statistical analyses were performed using SPSS version 23.0 (SPSS Inc., IBM, Chicago, IL, USA). The continuous data are presented as the mean±standard deviation or medians (ranges), and the categorical data are expressed as the absolute and relative frequencies. The continuous variables were analyzed using a Student’s t-test. The categorical data were examined using a Fisher’s exact test or chi-square test with a Yates’s correction.
6. Ethical considerations
The present study was conducted in accordance with the ethical guidelines of the Declaration of Helsinki. The Institutional review board of Chonnam National University Hospital approved this study (IRB No., CNUH-2018-199).
Go to :

DISCUSSION
In this study, Yamada type II lesions and central dimpling were the common gross endoscopic findings for GEP. The lesions were most commonly located in the antrum. The common EUS findings for GEP were mixed echogenicity, heterogeneous homogeneity, absence of calcification, and indistinct borders.
Although the typical endoscopic findings showed a submucosal nodule with central umbilication that corresponded to a draining duct, GEP has several endoscopic findings that can be confused with other SELs with malignant potential. Central dimpling, a characteristic endoscopic finding of GEP, was noted in 48.4% of the patients, which was similar to the results of previous studies.
4,12
The EUS findings of GEP are extremely diverse, and the inter-observer agreement was poor
13,14 because of the varying incidence of different pathological types of GEP.
14 The histological structure is similar to that of normal pancreatic tissue. Matsushita et al.
15 suggested the EUS findings of GEP with histological comparisons. An indistinct border was related to the lobular structure of the acinus tissue at the margin. The observed heterogeneous internal echo patterns were due mainly to hypoechoic acinus tissue accompanied by scattered small hyperechoic areas related to the adipose tissue content. A distinct margin and more hypoechoic features are related to the dense acinus tissue located predominantly in the PM. The anechoic echogenicity of GEP was associated with ductal dilatation of the pancreatic tissue. The thickening in the fourth layer was related to the hypertrophy of the PM.
Hase et al.
16 initially proposed a classification of the EUS findings of GEP. M-type lesions include lesions involving the PM layer. In the S-type, however, the ectopic pancreatic tissue is located in the submucosal and mucosal layers without involvement of the muscle layer. Park et al.
4 modified Hase’s classification such that the lesions were classified as the S-type and D-type. In the present study, D-type GEP was observed in 29% of patients. This finding was inconsistent with those of Park’s study,
4 because patients eligible for endoscopic resection were included. While S-type lesions are usually small and found in the antrum, D-type lesions are large and found in the gastric body.
17 In the present study, among nine patients with D-type lesions, seven lesions were relatively small (<15 mm in diameter) and located in the antrum. Sometimes, it may be difficult to diagnose D-type GEP because it appears as a heterogeneously hypoechoic mass involving the third and fourth layers on EUS such that it may be misdiagnosed as a gastrointestinal stromal tumor. Therefore, understanding and recognizing the common EUS findings of GEP and adequate tissue sampling may help avoid unnecessary surgery. The pathological diagnosis of GEP using conventional biopsy forceps is usually difficult because of the limitation of approaching deep specimens. Therefore, clinicians must decide whether endoscopic procedures, such as an endoscopic resection and EUS-guided intervention for tissue sampling, should be performed.
6,15,18-21 Endoscopic resection is a useful tool for the tissue sampling of gastric SELs,
7,12,22 particularly S-type lesions.
In some cases, however, it is difficult to achieve a complete resection because of noninflammatory adhesion to the deep layer, as explained by the aberrant primordium theory, in which during embryonic development, partial primordial pancreatic tissue forms noninflammatory adhesion with the adjacent gastric and intestinal wall and mesentery.
10 PM layer thickening is commonly observed in GEP. Even if the lesion does not show PM layer involvement, lesions with PM thickening are not subjected to a complete resection due to adhesion by pancreatic juice; therefore, the researchers analyzed this further. The expectation was that the complete ablation rate would decrease with PM thickening, but the results were different. Although the PM thickening area has adhesion by pancreatic juice, this adhesive tissue does not appear to interfere with the endoscopic resection through fibrotic changes.
An attempt was made to evaluate the EUS findings as predictors for a complete resection of GEP in this study. On the other hand, none of the EUS findings, such as border distinctness, presence of homogeneous echogenicity, and PM thickening, were significantly associated with a complete resection. Moreover, no residual lesions were detected in the follow-up despite the incomplete resection. Owing to the electrocautery effect of endoscopic ablation, the residual tissue may not have been observed even if complete ablation was not possible. Moreover, it is also possible that the recurrence could not be confirmed because of the relatively short follow- up period. As an ectopic pancreas is often asymptomatic and located in the subepithelium, follow-up endoscopy may not be a useful method to confirm the recurrence of an ectopic pancreas. In addition, even if it was not resected completely, no remaining tissue was observed, meaning that a complete resection may be unnecessary. Rather, before performing the endoscopic resection, it is better not to perform the endoscopic resection if the EUS findings have been accurately analyzed and judged to be suitable for the GEP.
This study had several limitations. A single-center study with a retrospective design based on observational data was conducted. Therefore, there were limitations in analyzing the EUS findings of GEP located in the deep layer and obtaining information regarding the diagnostic accuracy of the EUS findings. In addition, selection bias may be present because only the histological findings of GEP were confirmed and analyzed on the endoscopic resection. The difficulty in performing procedures, such as an endoscopic resection, may have been underestimated. Moreover, in cases of typical GEP, endoscopic removal was not performed. Nevertheless, these findings provide an impetus for future prospective studies with an accurate methodological design on endoscopic resection with respect to the diagnosis of GEP based on the EUS findings. EUS can help identify the features of GEP. Unnecessary removal of GEP can be avoided with careful observations of the EUS findings.
Go to :






 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download