INTRODUCTION
Autoimmune pancreatitis (AIP) is a treatable type of chronic pancreatitis that is characterized by its unique clinical, histological, and radiological features, including serum IgG4 elevation.
1 Although AIP responds well to steroids, the natural course of AIP is unclear, particularly regarding the association of AIP with pancreatic cancer. A few case reports have documented the synchronous
2-4 and metachronous
5-7 development of pancreatic adenocarcinomas or related cancer in patients with AIP. On the other hand, there are no case reports of AIP progressing to peritoneal carcinomatosis in patients who did not undergo surgery or steroid treatment. This paper reports a case of an untreated patient with type I AIP who developed peritoneal carcinomatosis without any obvious changes in the pancreas after more than 2 years of follow-up.
Go to :

CASE REPORT
A 66-year-old woman visited the outpatient clinic due to pancreatic abnormalities on an abdominal CT scan that had been performed in the Department of Urology to evaluate renal cysts. Abdominal CT scanning revealed diffuse enlargement with heterogeneous enhancement and surrounding infiltration in the pancreas (
Fig. 1A, B). The patient had no significant symptoms or a history of weight loss. A physical examination revealed a soft and flat abdomen. The initial laboratory tests showed no abnormal results, including the serum AST, ALT, amylase, and lipase levels. Additional laboratory tests revealed normal levels of CA 19-9 (8.7 U/mL, normal <37 U/mL) and elevated levels of IgG (2,167 mg/dL, normal <1,600 mg/dL) and IgG4 (849 mg/dL, normal <157 mg/dL). Abdominal MRI revealed an infiltrative mass-like lesion in the pancreatic head that encased the superior mesenteric artery and superior mesenteric vein with an extension along the mesenteric root. MRCP showed multiple strictures of the main pancreatic duct without marked upstream dilatation (
Fig. 1C). PET-CT demonstrated diffuse 18F-fluorodeoxyg- lucose uptake in the pancreas and distal common bile duct involving the peripancreatic, retropancreatic, splenic hilar, omental, and mesenteric areas. A transabdominal ultrasound-guided biopsy was performed on the pancreatic head lesion. The histology revealed storiform fibrosis, periductal lymphoplasma-cytic infiltration, obliterative phlebitis, and abundant IgG4-positive cells (
Fig. 1D-G).
 | Fig. 1At the initial diagnosis, (A, B) computed tomography revealed the diffuse enlargement of the pancreatic head and body with peripancreatic infiltration, and (C) magnetic resonance cholangiopancreatography shows multiple strictures of the main pancreatic duct (arrow) without marked upstream dilatation. Histopathological findings of the pancreatic head showing (D) storiform fibrosis, (E) periductal lymphoplasmacytic infiltration, (F) obliterative phlebitis (D-F: H&E, ×400), and (G) positive immunoglobulin G4 staining (×400). 
|
Based on the imaging findings, serum IgG4 levels, and biopsy results, the patient was diagnosed with type I AIP according to the international consensus diagnostic criteria.
8 After diagnosis, the patient was given 40 mg of prednisolone per day. On the third day after starting prednisolone, the patient visited the emergency room with dizziness and vomiting. Acute infarctions in both cerebella were diagnosed, and the prednisolone therapy was discontinued. After recovering completely from the infarctions, the patient refused to take prednisolone. Fourteen months after diagnosis, a follow-up abdominal CT scan revealed some resolution of the peripancreatic infiltration, but the prominent pancreatic enlargement of the pancreatic head with diffuse narrowing of the superior mesenteric vein and splenic vein and the collateral vessels remained (
Fig. 2). The serum CA 19-9 level was 72.5 U/mL at 14 months. Twenty-seven months after the diagnosis of AIP, the patient presented with epigastric pain. An abdominal CT scan revealed no obvious interval changes in the pancreas, showing enlargement of the pancreatic head and some infiltration around the pancreas (
Fig. 3A, B). On the other hand, some ascites with peritoneal nodularity was observed (
Fig. 3C).
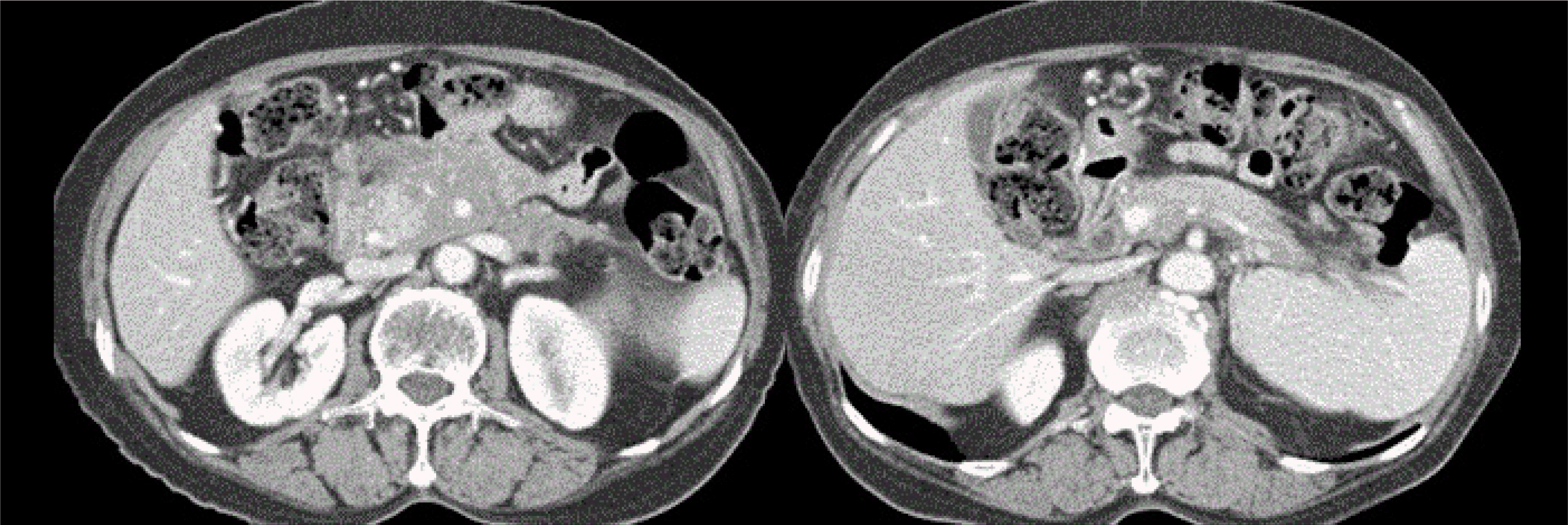 | Fig. 2Abdominal computed tomography (CT) findings at 14 months. A CT scan revealed some resolution of the peripancreatic infiltration, but there was still prominent pancreatic enlargement of the pancreatic head with a diffuse narrowing of the superior mesenteric vein and splenic vein and the collateral vessels. 
|
 | Fig. 3At 27 months, (A, B) abdominal computed tomography revealed no obvious change in the pancreas during follow-up, but (C) peritoneal nodularities (arrows) were identified. (D) The laparoscopy shows multiple whitish nodules on the peritoneum. (E, F) The histopathology of the nodules shows well-differentiated adenocarcinoma of a pancreaticobiliary origin (E: H&E, ×100; F: cytokeratin-7, ×200). 
|
The serum IgG4 level was similarly high (950 mg/dL), but the serum CA 19-9 had increased markedly to 2,756 U/mL. Diagnostic laparoscopy revealed multiple whitish nodules on the peritoneum (
Fig. 3D). A biopsy at the whitish nodules was performed, but no biopsy from the pancreas was done. The biopsy of the nodules showed a well-differentiated adenocarcinoma that was positive for cytokeratin-7 and caudal-related homeobox transcription factor (CDX)-2 and negative for cytokeratin-20. The results were compatible with adenocarcinoma of a pancreaticobiliary origin (
Fig. 3E, F). The patient was treated with gemcitabine and supportive therapy but died 33 months after the diagnosis of AIP due to the progression of cancer (
Fig. 4).
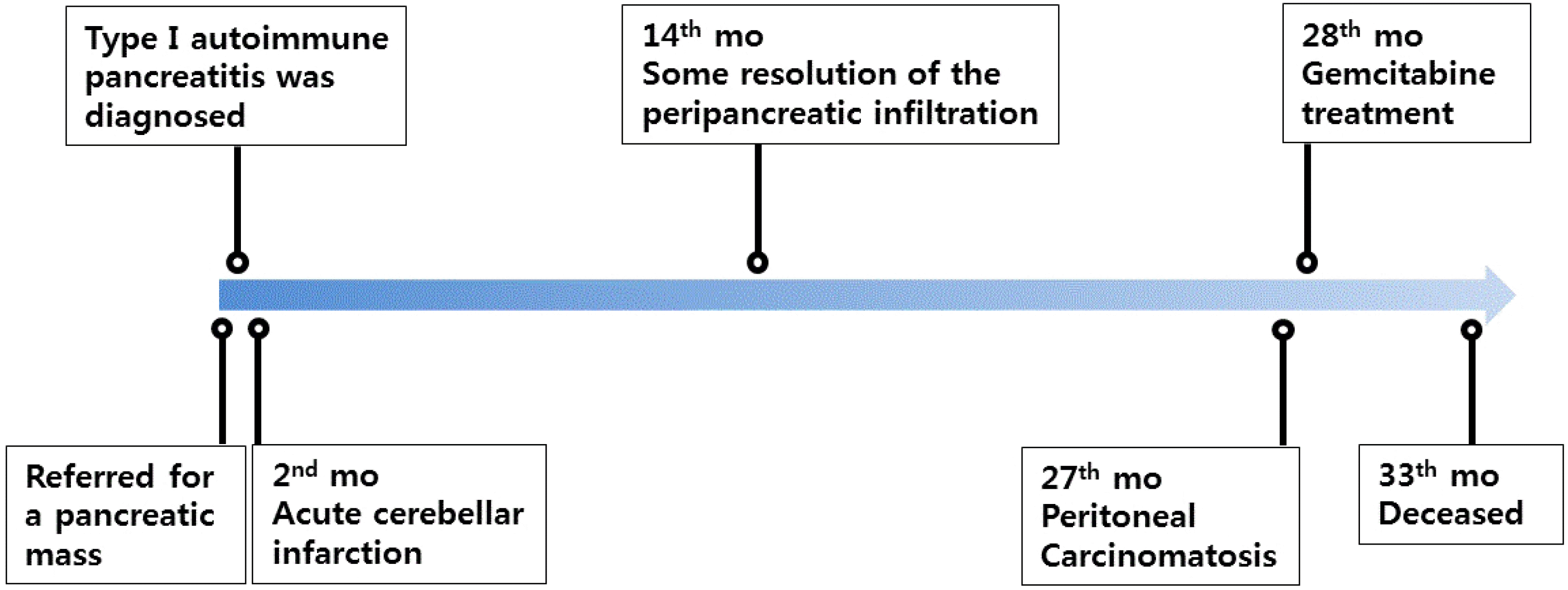 | Fig. 4Timeline of patient events. mo, months. 
|
Go to :

DISCUSSION
The association between AIP and pancreatic cancer is unclear. As a special type of chronic pancreatitis, AIP may also increase the risk of pancreatic cancer. In a recent meta-analysis, patients with chronic pancreatitis had an almost eight-fold increased risk of developing pancreatic cancer 5 years after the diagnosis.
9 In addition, a continuous active inflammatory status may advance the occurrence of malignancy.
10 AIP, which is characterized by prominent chronic inflammation, also increases the predisposition to pancreatic cancer.
5 The incidence of developing pancreatic cancer during the long-term follow-up ranges from 0% to 4.8%.
11 On the other hand, there has been little research on the association between AIP and pancreatic cancer. The lack of studies is due partly to the low incidence of AIP and pancreatic cancer in the general population. Several studies have documented the synchronous development of pancreatic cancer and AIP.
2-4 In those cases, it was impossible to show a causal relationship between AIP and pancreatic cancer. This synchronous development can be either an incidental or a cause and effect relationship.
In contrast, metachronous pancreatic cancer can occur during the follow-up of AIP patients. Thus far, 11 cases (case reports
5-7 and original articles
12-15) of the metachronous development of pancreatic cancer and AIP have been reported (
Table 1). All the patients reported had type I AIP, and the intervals between AIP and cancer diagnosis varied widely, ranging from 9 to 186 months. Among the 11 cases, five patients showed a slightly elevated serum IgG4 levels, and one showed marked elevation. Four patients had increased IgG4-positive plasma cells in the pancreatic tissues. The present case showed that the serum IgG4 level was markedly elevated (849 mg/dL), with also abundant IgG4-positive cells in the tissue. Three patients showed carcinomatosis, as in the present case. Two cases
5,14 advanced to pancreatic body cancer with carcinomatosis approximately 5 years later. One case
7 developed peritoneal carcinomatosis 1 year later. The present patient was diagnosed with peritoneal carcinomatosis 27 months after the diagnosis of AIP, interestingly, without any obvious change in the pancreas. Unlike typical pancreatic ductal adenocarcinoma, the pancreas in the present case changed little over the 27 months, even with the development of peritoneal carcinomatosis. AIP tends to cause retroperitoneal infiltration.
16 Hence, this case highlights the potential of a peritoneal metastasis via the retroperitoneal space without apparent changes in the pancreas.
Table 1
Reported Cases of AIP with Metachronous Development of Pancreas-related Cancer
|
Cases |
Age/sex |
IgG4 serum |
IgG4 tissue |
Pancreatic surgery |
Steroid therapy |
Intervala (mo) |
Site |
|
Ghazale and Chari (2007)5
|
72/M |
Unknown |
>30/HPF |
PD |
None |
60 |
Body, carcinomatosis |
|
Fukui et al. (2008)6
|
80/M |
154 mg/dL |
Unknown |
None |
36 mo |
36 |
Body |
|
Loos et al. (2011)7
|
67/F |
Normal |
>10/HPF |
None |
1 mo |
12 |
Carcinomatosis |
|
Gupta et al. (2013)12
|
73/M |
Unknown |
>50/HPF |
PD |
None |
120 |
Tail, 2 cm |
|
69/M |
147 mg/dL |
16/HPF |
None |
72 mo |
96 |
Head and neck |
|
Hart et al. (2013)15
|
?/M |
>2 UNL |
Unknown |
PR |
Unknown |
9 |
Pancreas |
|
Hirano et al. (2014)13
|
58/M |
220 mg/dL |
Unknown |
Unknown |
Yes |
119 |
Pancreas |
|
70/M |
875 mg/dL |
Unknown |
Unknown |
None |
162 |
Pancreas |
|
Ikeura et al. (2018)14
|
61/F |
338 mg/dL |
Unknown |
PD |
31 mo |
31 |
Head |
|
39/F |
Unknown |
Unknown |
DP |
186 mo |
186 |
Body |
|
80/M |
154 mg/dL |
Unknown |
None |
67 mo |
67 |
Head, carcinomatosis |

Endoscopic ultrasound (EUS) was not performed to evaluate AIP in this case. Although EUS is not currently in the diagnostic algorithm for AIP, it has a promising role in the diagnosis of this disorder because of its ability to provide high-quality images and obtain tissue samples.
17 In addition, as new EUS tissue acquisition needles are used increasingly, the ease of obtaining core biopsies may make EUS an important diagnostic tool for a histology confirmation of AIP.
The present patient was not treated with steroids because of the cerebellar infarction and their reluctance. Whether the continuous steroid treatment of AIP patients can prevent pancreatic cancer development is unclear. A lack of or discontinuation of steroid treatment can affect the development of pancreatic cancer, but this requires further study.
The coexistence of pancreatic cancer at the time of the initial diagnosis in cases of metachronous cancer development is controversial. In the present patient, the CT scan showed no changes until 14 months. On the other hand, the CA 19-9 level increased slightly (72.5 U/mL) at that time. Therefore, a precursor or very small lesions of pancreatic cancer may have coexisted in AIP at the initial presentation. Gupta et al.
12 reported 23 cases (82%) of pancreatic intraepithelial neoplasia in 29 AIP patients who underwent pancreatic resections. This suggests that AIP may have precancerous lesions and the potential to develop a malignancy.
In summary, this case describes the progression of an untreated type I AIP into peritoneal carcinomatosis without any obvious changes in the pancreas for more than 2 years. Peritoneal carcinomatosis may occur during the follow-up of patients with AIP, even without any noticeable changes in the pancreas. Whether the continuous medical treatment of AIP can prevent the development of pancreatic cancer requires further clarification.
Go to :








 PDF
PDF Citation
Citation Print
Print




 XML Download
XML Download